Ant Group Announces Major Upgrades to Its 15-Million-MAU AI Health App AQ Amid Wider Push into Health Sector
Ant Group today announced major upgrades to its AI health app AQ, with a new Chinese name “Ant A-Fu.” The character “Fu” conveys good wishes and well-being, reflecting the app’s goal to promote better health and quality of life.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20251215203213/en/

Ant Group’s AI health app AQ features three core capabilities: health Q&A, AI health companion, and integrated health services.
The upgraded version of AQ now features three core capabilities: health Q&A, AI health companion, and integrated health services.
Launched in June 2025, AQ has rapidly grown to 15 million monthly active users, making it China’s leading AI health management app. Positioned as an AI health companion service, AQ currently answers over 5 million health-related questions every day.
The announcement comes amid China’s ongoing demographic shift. According to projections from the National Health Commission, by around 2035, more than 400 million people, or over 30% of the population, will be aged 60 or above. This transformation is expected to significantly increase demand for health-related services, with AI-powered solutions playing a critical role in meeting those needs.
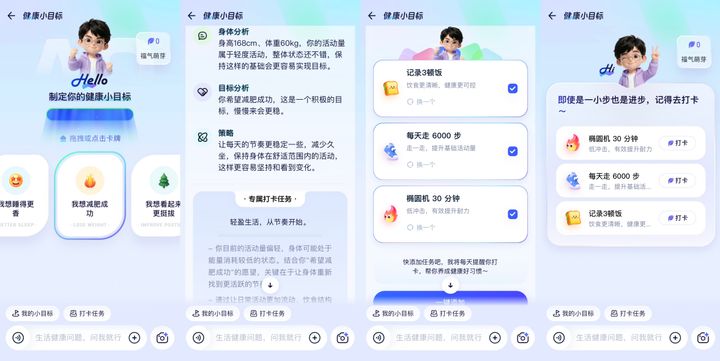
Building Healthier Habits with an AI Health Companion
The newly launched health companion feature is designed to help users develop and maintain healthy habits.
-
Users can log their health status directly in AQ. The app now also supports records integration from major smart device brands, such as Apple, Huawei, vivo, Yuwell, and Omron. With user consent, AQ can automatically sync daily health metrics—such as physical activity, heart rate, and sleep duration—from these devices.
Beyond smart devices, users can also upload photos or images to record health information. AQ allows users to create health profiles for family members as well. Acting much like a family doctor, the app can track long-term health trends and help safeguard the well-being of the entire household.
- Second, AQ introduces two new companion tools: health goal setting and smart reminders. Users can set goals related to exercise, diet, or lifestyle habits, while AQ functions like a personal trainer—creating tailored plans and sending daily reminders to help users stay on track.
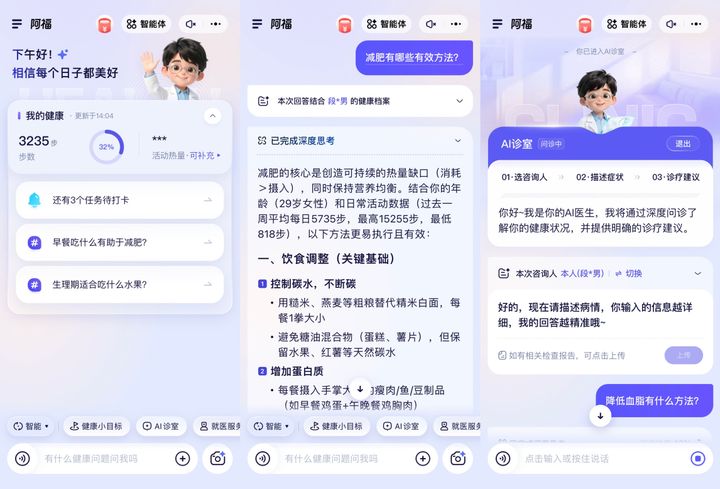
More Personalized Health Q&A
With a deeper understanding of users’ health situations, AQ’s health Q&A function now delivers more accurate and personalized responses.
- For users who find it difficult to clearly describe their symptoms, AQ’s “AI Clinic” tool simulates a real face-to-face consultation by proactively asking follow-up questions and offering visual prompts to help users express their concerns more precisely.
- In addition to voice and text, AQ can also analyze images. Users can take photos of skin conditions, medical test reports, or medication packaging to receive clear explanations, with the capability of interpreting most medical reports.
AQ does not provide medical diagnoses or replace professional doctors. As Zhang Junjie, Vice President of Ant Group and Head of Health Business, explained: “AQ is designed to help users address common, everyday health questions and support healthy habit formation, meeting users’ daily health needs that don’t yet require a hospital visit.”
For cases requiring medical diagnosis, AQ connects users with a nationwide network of 300,000 doctors for online consultations. Users can also book in-person doctor appointments, purchase medication, and pay hospital bills using their basic medical insurance e-certificates directly through the app.
AQ has also partnered with over 500 leading physicians across China to launch their AI Doctor Agents on the platform, which provide health recommendations by learning from each doctor’s prior experience. Together, these agents have answered more than 27 million health-related inquiries.
By handling routine and everyday health questions, AQ allows doctors to focus more of their time on treating complex conditions. “AQ can serve as a powerful assistant to hospitals and physicians,” Zhang added. “By leveraging digital platforms and intelligent technology, we can enable one doctor to serve more patients, making high-quality health services more accessible and inclusive.”
About Ant Group
Ant Group is a global digital technology provider and the operator of Alipay, a leading internet services platform in China, connecting over one billion users to more than 10,000 types of consumer services from partners. Through innovative products and solutions powered by AI, blockchain and other technologies, Ant Group supports partners across industries to thrive through digital transformation in an ecosystem for inclusive and sustainable development. For more information, visit www.antgroup.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20251215203213/en/

Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Celltrion receives positive CHMP Opinion for SteQeyma™ (ustekinumab biosimilar) autoinjector18.12.2025 03:41:00 CET | Press release
SteQeyma™45mg and 90mg solution for injection via autoinjector (pre-filled pen) receives positive CHMP opinion, which will facilitate subcutaneous administration in patients with plaque psoriasis, psoriatic arthritis (PsA) and Crohn’s disease (CD)1The new autoinjector option increases convenience, enhances individual patient experience and expands administration options Celltrion, Inc. today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion of autoinjector of SteQeyma™, a biosimilar to Stelara® (ustekinumab), for the treatment of plaque psoriasis, psoriatic arthritis (PsA) and Crohn’s disease (CD). The positive CHMP opinion is for SteQeyma autoinjector in 45mg/0.5mL and 90mg/1mL, expanding the currently approved SteQeyma™ presentation, which includes 45mg/0.5mL, 90mg/1mL in a pre-filled syringe and 45mg/0.5mL in a vial for subcutaneous injection, as well as 130mg/26mL concentrate for solution f
Megaport Expands into India, Accelerating Global Growth with Extreme IX Acquisition18.12.2025 02:15:00 CET | Press release
Through the Extreme Exchange (IX) acquisition, Megaport gains seven Internet Exchanges and access to 40+ data centres across India’s fastest-growing digital hubs. Megaport Limited (ASX: MP1) (“Megaport”), the world’s leading Network as a Service (NaaS) provider, today announced the acquisition of Extreme IX,India’s leading Internet Exchange operator, from Extreme Labs, a Bulgaria-headquartered software and network engineering company that incubated the Extreme IX platform. The acquisition expands Megaport’s global platform into one of the world’s fastest-growing digital infrastructure markets and supports the Company’s strategy to deliver scalable, high-performance connectivity services across APAC. The acquisition establishes Megaport’s presence across seven Internet Exchanges in major Indian metros: Delhi, Kolkata, Hyderabad, Chennai, Bengaluru, Mumbai, and Pune, connecting 40+ data centres and more than 400 customers. It also accelerates Megaport’s planned market entry by nearly thr
IonQ and QuantumBasel Expand Long-Term Partnership in Next-Generation Quantum Systems17.12.2025 22:10:00 CET | Press release
Extension solidifies QuantumBasel as IonQ’s Innovation Center in Europe; adds IonQ Tempo and next-generation system to advance quantum commercialization IonQ (NYSE: IONQ), the world’s leading quantum company, today announced an expanded agreement with QuantumBasel, the quantum initiative of uptownBasel, Switzerland’s innovation campus. The extended contract grants QuantumBasel ownership of its existing IonQ Forte Enterprise system and secures ownership of a next-generation Tempo system. This new agreement brings the total deal value of the QuantumBasel and IonQ partnership to over $60 million and extends IonQ’s on-site presence in Switzerland four more years, continuing through 2029. QuantumBasel is IonQ’s official Innovation Center in Europe, serving as a hub for European industry, academia, and research institutions to explore practical quantum computing applications and access IonQ’s latest enterprise-grade systems. “Our extended partnership with QuantumBasel represents a cornerston
Suzano Starts Up New Production Line, Boosting Its Fluff Pulp Capacity by 400%17.12.2025 21:50:00 CET | Press release
A R$490 million investment expands the supply of raw material used in the production of absorbent items Suzano, the world’s largest pulp producer, has commenced operations this week at its new fluff pulp production line located in its Limeira unit in Brazil’s São Paulo state. This R$490 million investment increases Suzano’s total fluff pulp production capacity by more than 400%, from 100,000 to 440,000 tonnes per year. The project involved converting the existing pulp line at the Limeira unit into a flexible machine, capable of producing both Eucafluff® and market pulp. Eucafluff® is used in the production of absorbent and personal hygiene products, such as baby and adult diapers, sanitary pads and pet pads. Then market pulp is supplied for making products including toilet paper, printing and writing papers, and paper packaging. Launched in 2015, Eucafluff® is the world’s first fluff pulp made from eucalyptus, delivering unique advantages like enhanced softness and flexibility, which t
SES Acknowledges Moody’s Rating Action and Reiterates Deleveraging Commitments17.12.2025 21:36:00 CET | Press release
SES S.A. (“SES” or the “Company”), a leading space solutions company, acknowledges the credit rating action announced by Moody’s Investor Service today, which follows the release of SES’ Q3 2025 results and Intelsat integration update. SES management reiterates that the Company continues to execute on its strategy with a clear plan to strengthen its key credit metrics over time. Consistent with this plan, it remains management’s intention to de-lever and return to credit metrics that are commensurate with investment grade, with a policy objective of reducing adjusted net leverage1 to at least 3.0x or below. Today’s rating action does not change the Company’s ability to operate its business, serve customers, or execute its strategic plan. SES maintains a balanced weighted average debt maturity profile of approximately five years, and the rating action from Moody’s is not expected to have a material impact on the interest payable under the Company’s existing debt facilities. SES also ben
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom