New Study Finds Masimo SedLine® Brain Function Monitoring Offers Significant Advantages During Pediatric Anesthesia
EEG-Guided Anesthesia Using Masimo Technology Significantly Reduced Pediatric Anesthesia Emergence Delirium, Time to Emergence and Discharge, and Cost of Care by Minimizing Sevoflurane Exposure
Masimo (NASDAQ: MASI) today announced the findings of a randomized clinical trial published in JAMA Pediatrics in which Dr. Yasuko Nagasaka and colleagues at Tokyo Women’s Medical University demonstrated the ability of electroencephalogram (EEG)-guided anesthesia, using Masimo SedLine® Brain Function Monitoring, to improve anesthesia administration in children undergoing surgery.1 The researchers found that the use of SedLine led to a significant reduction in the amount of a commonly used inhalation anesthetic (sevoflurane) needed to maintain anesthesia in pediatric patients, reducing their exposure to the drug. In turn, the patients experienced a significantly lower rate of pediatric anesthesia emergence delirium, or PAED, which commonly manifests in confusion, agitation, or hallucinations experienced during recovery. When compared with standard practice, children monitored with SedLine also regained consciousness faster and could be safely discharged to the post-anesthesia care unit, or PACU, sooner – time savings that, the researchers noted, may represent additional cost savings. In sum, the researchers found that Masimo SedLine helped to make pediatric anesthesia safer, more efficient, and more cost effective.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250610391732/en/
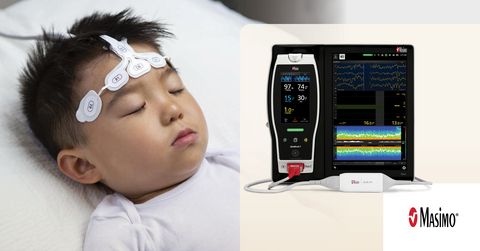
Masimo SedLine® Brain Function Monitoring
Children monitored with SedLine were exposed to an average of 1.4 MAC hours less sevoflurane and had a 14% lower incidence rate of PAED. On average, they regained consciousness 53% sooner and were discharged from the PACU 48% sooner. The time saved led to an estimated cost savings of $240 to $920 per patient.
PAED is a frequent and clinically significant complication in pediatric anesthesia,2 often leading to short-term distress among pediatric patients, parents, and staff. Some of the negative postoperative behaviors can persist for weeks or months.3 Exposure to commonly used volatile anesthetics like sevoflurane gas has been shown to contribute to PAED.4 In current standard practice, a fixed amount of sevoflurane, 1.0 minimum alveolar concentration (MAC), is used to maintain pediatric anesthesia, but as the authors note, that may be excessive.5 Dr. Nagasaka and her team hypothesized that by using bilateral EEG – in particular, the waveforms and multitaper density spectral array (DSA) spectrograms displayed by Masimo SedLine – to guide sevoflurane titration, the amount needed to induce and maintain appropriate sedation could be reduced, thereby reducing the incidence of PAED.
In the study, children aged 1 to 6 years scheduled for elective surgery involving at least 30 minutes of general anesthesia at the largest children’s hospital in Japan were randomly assigned to an experimental group (n=91), whose anesthesia was guided by Masimo SedLine, or a control group (n=86), whose anesthesia was guided using standard practice, i.e. a fixed sevoflurane dosage of 1.0 MAC. The researchers recorded the amount of sevoflurane exposure for each patient and the time elapsed between important events including intubation, extubation, arrival and discharge from the PACU, and emergence from anesthesia. All patients were assessed for PAED by clinicians blinded to the treatment assignment, using a standard scale.
The researchers found that the incidence of PAED was significantly lower among patients in the SedLine group, affecting 21% of patients vs. 35% of patients in the control group (p=0.04). SedLine group patients were exposed to significantly less sevoflurane, by an average 1.4 MAC hours. They were extubated sooner, by an average of 3.3 minutes, regained consciousness sooner, by an average of 21.4 minutes, and were discharged from the PACU sooner, by an average of 16.5 minutes. The researchers estimated that the reduction in time spent in the OR and PACU, of approximately 20 minutes, may represent a cost savings of $240 to $920 (USD) per case.
The authors concluded, “EEG-guided management of general anesthesia reduced sevoflurane exposure and pediatric anesthesia emergence delirium in children, with faster emergence and shorter post-anesthesia care unit stays. The findings suggest that high concentrations of sevoflurane for induction followed by routine use of 1.0-MAC sevoflurane for maintenance may be excessive.”
They also noted that “with EEG monitoring, parents and guardians may be reassured that health care professionals can make an active effort to reduce and minimize a child’s exposure to anesthetic drugs.”
A similar study in 2022 by Long et al. – one of the first to investigate the impact of EEG-guided anesthesia on children undergoing surgery – found that pediatric patients monitored with SedLine experienced significantly fewer EEG patterns of profound brain inactivity, known as burst suppression. Burst Suppression has also been associated with adverse outcomes, including postoperative delirium.6
Yasuko Nagasaka, Professor of Anesthesia at Tokyo Women’s Medical University and the new study’s senior author, commented, “While general anesthesia is necessary for pediatric patients undergoing surgery, parents and guardians may express concerns about their child’s exposure to anesthetic drugs. We can now offer reassurance by explaining that modern medical technologies, such as EEG-guided anesthesia care, help minimize anesthetic exposure, potentially reducing the incidence of pediatric emergence delirium (PAED) and supporting earlier awakening and recovery from unconsciousness.”
“It is important to recognize,” continued Dr. Nagasaka, “that increasing the depth of anesthesia is relatively easy to learn. On the other hand, developing the confidence and skill to safely reduce anesthesia requires deeper understanding. Our results may offer a great step forward towards the improvement of our common practice, which may lead to the development of guidelines for EEG monitoring in pediatric areas to eventually mandate EEG monitoring during general anesthesia in the future. But comprehensive training in EEG-guided anesthesia should be considered a critical next step for the entire anesthesia community.”
Dean Kurth, MD, attending anesthesiologist at Children’s Hospital of Philadelphia and Professor of Anesthesiology and Critical Care Medicine at the University of Pennsylvania Perelman School of Medicine, added, “This study by Miyasaka et al. confirms the growing realization by pediatric anesthesiologists worldwide that more anesthesia drug is being administered to children than necessary, which has negative consequences. As the authors have shown, SedLine EEG data can help clinicians provide more precise anesthetic drug dosing for each child and improves outcomes.”
@Masimo | #Masimo
References
- Miyasaka K, Suzuki Y, Brown E, Nagasaka Y. EEG-Guided Titration of Sevoflurane and Pediatric Anesthesia Emergence Delirium. JAMA Pediatrics. April 21, 2025. DOI: 10.1001/jamapediatrics.2025.0517.
- Cole JW, Murray DJ, McAllister JD, Hirshberg GE. Emergence behavior in children: defining the incidence of excitement and agitation following anaesthesia. Paediatr Anaesth. 2002;12(5):442-447. DOI:10.1046/j.1460-9592.2002.00868.x.
- Davis L, Qi TS, and Ng A. Emergence delirium: an overview with an emphasis on the use of electroencephalography in its management. Anest Pain Med. 2024;19(Suppl 1):S87-95. DOI: 10.17085/apm.24013.
- Mason KP. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. 2017;118(3):335-343. DOI: 10.1093/bja/aew477.
- Goddard N, Smith D. Unintended awareness and monitoring of depth of anaesthesia. Contin Educ Anaesth Crit Care Pain. 2013;13(6):213-217.
- Long MHY, Lim EHL, Balanza GA, Allen JC, Purdon PL, and Bong CL. Sevoflurane requirements during electroencephalogram (EEG)-guided vs. standard anesthesia care in children: A randomized controlled trial. J Clin Anesth. 27 Jun 2022. DOI: https://doi.org/10.1016/j.jclinane.2022.110913.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve life, improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown to outperform other pulse oximetry technologies in over 100 independent and objective studies, which can be found at www.masimo.com/evidence/featured-studies/feature. Masimo SET® is estimated to be used on more than 200 million patients around the world each year and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2025 Newsweek World’s Best Hospitals listing. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements other than statements of historical facts that address activities, events or developments that we expect, believe or anticipate will or may occur in the future. These forward-looking statements include, among others, statements regarding the performance of SedLine to achieve certain results including the incidence of pediatric emergence delirium, earlier awakening, recovery from unconsciousness, or cost savings; the rate of adoption of SedLine technology by pediatric anesthesiologists for use during anesthesia administration in children undergoing surgery; and other matters that do not relate strictly to historical facts or statements of assumptions underlying any of the foregoing. These statements are often identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “on-going,” “opportunity,” “plan,” “potential,” “predicts,” “forecast,” “project,” “seek,” “should,” “will,” or “would,” the negative versions of these terms and similar expressions or variations, but the absence of such words does not mean that a statement is not forward-looking. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: the highly competitive nature of the markets in which we sell our products and technologies; the ability to obtain regulatory approvals; the lack of acceptance of any of our current or future products and technologies; and other factors discussed in the “Risk Factors” section of our most recent periodic reports filed with the Securities and Exchange Commission (“SEC”), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC’s website at www.sec.gov. Forward-looking statements are not guarantees of future performance. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250610391732/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Transition Industries Signs Strategic Agreements for the Pacifico Mexinol Project, the Largest Standalone Ultra-Low Carbon Chemical Production Facility in the World30.6.2025 20:30:00 CEST | Press release
Pacifico Mexinol project, a 6,130 MT per day ultra-low carbon methanol production facility worth more than US$3.3b will be located near Topolobampo, Ahome, Sinaloa. Once operational in 2029, Pacifico Mexinol is poised to be the largest standalone ultra-low carbon chemical production facility in the world. Transition Industries LLC, a developer of world-scale, net-zero carbon emissions methanol and green hydrogen projects in North America, held a signing event for an Engineering, Procurement, and Construction (EPC) contract with the consortium of Samsung E&A Co., Ltd. (Samsung E&A), Grupo Samsung E&A Mexico, S.A. de C.V., and Techint Engineering and Construction for the Pacifico Mexinol project located in Ahome, Sinaloa, Mexico, which is contingent upon the fulfillment of customary conditions precedent and obtainment of all required approvals. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250630940954/en/ MAIRE group’s techn
Westinghouse and ITER Sign a $180M Contract to Advance Nuclear Fusion30.6.2025 15:45:00 CEST | Press release
The contract includes the assembly of the fusion reactor’s vacuum vessel, a key milestone which gets the project closer to replicating fusion energy on Earth Westinghouse Electric Company and ITER signed a contract for $180 million for the assembly of the vacuum vessel for the fusion reactor. This is a key milestone in the construction of the ITER reactor, leading the way toward the use of fusion as a practical future source of reliable carbon-free energy. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250630497810/en/ The ITER Tokamak pit with the two vacuum vessel sector modules installed. Westinghouse has participated in the fabrication of the sectors of the vacuum vessel, as part of the Fusion for Energy (F4E) Consortium with its partners Ansaldo Nucleare and Walter Tosto. Westinghouse will be responsible for completing the vacuum vessel which is ITER’s most critical component: a hermetically sealed, double-walled steel
Monetate Acquires SiteSpect to Deliver AI-Native Personalization and Testing at Enterprise Scale30.6.2025 15:00:00 CEST | Press release
Monetate’s Real-Time Personalization Unites with SiteSpect’s Zero-Flicker Testing to Optimize Digital Experiences with Unmatched Speed, Precision, and SecurityNow Global Ecommerce and Digital Experience Leaders Can Access a Best-in-Class, Enterprise-Grade Personalization, Testing, and Optimization Platform Monetate, the leading AI-driven personalization platform, today announced it has acquired SiteSpect, a leader in A/B testing, to drive next-generation digital experience optimization. This acquisition accelerates Monetate’s vision to deliver intelligent, intentional, and individualized experiences at scale, powered by agentic AI and backed by the industry’s most advanced, enterprise-grade infrastructure. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250630514541/en/ The combination of Monetate’s real-time personalization and SiteSpect’s zero-flicker testing will yield an industry-first solution for enterprise-grade person
SS&C Blue Prism Recognized as a Gartner® Magic Quadrant™ RPA Leader for the Seventh Consecutive Year30.6.2025 15:00:00 CEST | Press release
SS&C Technologies Holdings, Inc. (Nasdaq: SSNC) today announced that SS&C Blue Prism has been recognized as a Leader in the 2025 Gartner Magic Quadrant for Robotic Process Automation (RPA). “We’re delighted SS&C Blue Prism has been named a Leader in the Gartner Magic Quadrant for Robotic Process Automation for the seventh year running,” said Bill Stone, CEO and Chairman of SS&C Technologies. “SS&C Blue Prism combines market-leading RPA and orchestration technologies with the latest artificial intelligence so organizations can tackle more complex tasks and dynamic business processes. We’ve scaled to more than 2,700 digital workers and AI agents across our own operations, resulting in over $200 million in annual savings. With SS&C leading the charge on deployment, customers can be confident in rolling out SS&C’s automation solutions securely, effectively, and responsibly.” More than 2,800 companies worldwide leverage SS&C Blue Prism for AI-powered automation, helping organizations delive
Takeda Announces U.S. FDA Approval of GAMMAGARD LIQUID ERC, the Only Ready-to-Use Liquid Immunoglobulin Therapy with Low Immunoglobulin A (IgA) Content130.6.2025 14:00:00 CEST | Press release
GAMMAGARD LIQUID ERC [immune globulin infusion (human)] with Less Than or Equal to 2 µg/mL IgA in a 10% Solution is Approved for Intravenous or Subcutaneous Use in People Aged Two and Older with Primary Immunodeficiency1U.S. Commercialization of GAMMAGARD LIQUID ERC Projected to Begin in 2026Company Announces Future Manufacturing Discontinuation End Date for Takeda's First-Generation Low-IgA Product, A Freeze-Dried Formulation in Company’s Differentiated Immunoglobulin Portfolio of Ready-to-Use Liquids2 Takeda(TSE:4502/NYSE:TAK) today announced that the U.S. Food and Drug Administration (FDA) has approved GAMMAGARD LIQUID ERC [immune globulin infusion (human)] with less than or equal to 2 µg/mL IgA in a 10% solution, the only ready-to-use liquid immunoglobulin (IG) therapy with low immunoglobulin A (IgA) content, as replacement therapy for people two years of age and older with primary immunodeficiency (PI). As a ready-to-use liquid, GAMMAGARD LIQUID ERC may help ease the administratio
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom