ASCO 2025: REVEAL GENOMICS Unveils New HER2DX Data From 800+ Patients
HER2DX analysis includes data from over 800 patients across four studies in the neoadjuvant (CompassHER2 pCR, BionHER), adjuvant (RESPECT) and metastatic (DFCI) settings.In neoadjuvant trials, HER2DX identified HER2-positive tumors more likely to respond to single taxane and dual HER2 blockade, and significantly contributed to predicting which patients would have a pathologic complete response (pCR) when added to clinical factors.In the adjuvant RESPECT study, HER2DX delivered robust prognostic information and predicted chemotherapy benefit in older adults (aged 70–80 years) with HER2-positive early breast cancer.In the metastatic setting, HER2DX ERBB2 signature score was significantly associated with survival outcomes following trastuzumab deruxtecan monotherapy.
REVEAL GENOMICS, S.L., a biotechnology company advancing precision oncology through biomarker innovation, today announced the presentation of four independent studies at the 2025 ASCO Annual Meeting. The studies, involving more than 800 patients, evaluated the clinical utility of its flagship test, HER2DX, in early-stage HER2-positive (HER2+) breast cancer.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250527779428/en/
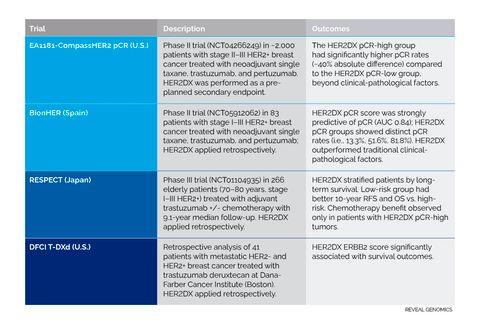
HER2DX studies presented at ASCO.
The four studies, CompassHER2 pCR, BionHER, RESPECT and DFCI, validate HER2DX as a powerful genomic tool for predicting response to therapy and long-term prognosis, addressing critical questions. The findings, which provide valuable insights for optimizing treatment strategies in early-stage HER2+ breast cancer and metastatic breast cancer, will be shared in oral and poster sessions led by academic investigators from the U.S., Europe, and Japan.
CompassHER2 pCR or EA1181 (U.S.): HER2DX determining response to THP-based de-escalated therapy
This study is led by the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN), a scientific organization that designs and conducts cancer research involving adults who have or are at risk of developing cancer, comprising nearly 1,400 member institutions in the U.S. and around the world.
HER2DX was evaluated as a secondary aim as part of a pre-specified, prospectively planned analysis embedded in the CompassHER2 pCR trial, also known as EA1181 (NCT04266249). This large, prospective multicenter phase II trial is assessing neoadjuvant taxane, trastuzumab, and pertuzumab (THP) in more than 2,000 patients with stage II–III HER2+ breast cancer, without the use of anthracyclines, cyclophosphamide, and carboplatin. The HER2DX assay was defined as a key secondary endpoint, with profiling of all available baseline samples planned.
Among 569 patients assessed to date for the HER2DX pathologic complete response (pCR) score, those with higher scores achieved significantly greater pCR rates, independent of estrogen receptor (ER) status. These findings confirm the ability of HER2DX to identify tumors more likely to respond to THP. Importantly, additional results are expected from this trial, as patients continue to be followed for the primary endpoints of 3-year survival outcomes, and the study team plans to profile many more baseline samples.
“The ability to predict response to THP is extremely valuable,” said Dr. Nadine Tung, Director of Breast Medical Oncology at Beth Israel Deaconess Medical Center, Professor of Medicine at Harvard Medical School, and Principal Investigator of the CompassHER2 pCR (EA1181) trial. “HER2DX provides a biology-driven tool that contributes to the selection of patients who can safely reduce chemotherapy, something we’ve long needed in HER2-positive early breast cancer.”
“It is encouraging to see the assay’s performance in both ER-positive and ER-negative subgroups,” added Dr. Tung. “The plan is to profile all available samples from the trial, and these early data already provide valuable insights for guiding treatment selection.”
BionHER trial (Spain): Validation of HER2DX-guided de-escalation strategy
The BionHER phase II trial (NCT05912062) evaluated HER2DX in 83 patients with stage I–III HER2+ breast cancer treated with neoadjuvant THP for up to 15 weeks at the Catalan Institute of Oncology (ICO L’Hospitalet, Barcelona, Spain). HER2DX was successfully performed in all baseline FFPE tumor samples.
The HER2DX pCR score was significantly associated with pCR with a performance AUC of 0.835. Patients classified by HER2DX pCR score into low, medium, and high groups had increasing pCR rates of 13.3%, 51.6%, and 81.8%, respectively. HER2DX outperformed traditional biomarkers, such as ER, Ki-67, and tumor-infiltrating lymphocytes, in predicting pCR.
“HER2DX is transforming the way we approach treatment selection in HER2-positive disease,” said Dr. Sònia Pernas, head of the breast cancer unit at ICO L’Hospitalet, and Principal Investigator of the BionHER study. “By identifying tumors more likely to respond to dual HER2 blockade with a single taxane, we are increasingly able to personalize treatment intensity without compromising efficacy.”
RESPECT trial (Japan): a response for an unrepresented population
Trans-RESPECT is a prespecified translational analysis within the Japanese RESPECT non-inferiority trial (NCT01104935), in which 266 patients aged 70–80 years with stage I-III HER2+ breast cancer were randomized to receive adjuvant trastuzumab with or without chemotherapy (Sawaki et al. J Clin Oncol 2020). HER2DX was assessed in 154 of these patients, with a median follow-up of 9.1 years. The HER2DX risk score effectively stratified patients by 10-year relapse-free survival (86.6% in the low-risk group vs. 68.4% in the high-risk group) and overall survival (94.5% vs.72.5%). Notably, chemotherapy conferred an overall survival benefit only in tumors classified as pCR-high by HER2DX, with a significant interaction p-value of 0.045.
“HER2DX provided clear long-term prognostic information in this elderly population,” said Dr. Kazuki Nozawa, Lead Investigator of Trans-RESPECT, from the Department of Advanced Clinical Research and Development and the Department of Breast Surgery at Nagoya City University Graduate School of Medical Sciences. “Importantly, its ability to help identify which patients may benefit from adjuvant chemotherapy is highly relevant to clinical decision-making. Older patients with HER2DX low-risk scores, particularly those classified as pCR-low or -medium, appear to be ideal candidates for trastuzumab alone, without chemotherapy.”
DFCI T-DXd study (U.S.): predicting survival outcome to T-DXd
Dana-Farber Cancer Institute (DFCI) conducted a retrospective analysis of 41 patients with metastatic breast cancer (25 HER2-positive, 16 HER2-negative) treated with trastuzumab deruxtecan (T-DXd) monotherapy between 2017 and 2023. The HER2DX HER2 signature score was significantly associated with time to next treatment (p=0.001). When stratified into tertiles, patients in the highest tertile had a median time to next treatment of 12.03 months compared to 4.7 months in the lowest tertile (p=0.02).
“Quantitative measurement of HER2 amplicon expression by HER2DX is a key determinant of T-DXd activity in metastatic breast cancer,” said Dr. Sara M. Tolaney, Chief of the Division of Breast Oncology at Dana-Farber Cancer Institute. She added, “Further evaluation of this genomic test in ongoing T-DXd trials is warranted.”
HER2DX makes a significant impact at ASCO 2025
“ASCO 2025 marks a pivotal milestone for HER2DX,” said Dr. Aleix Prat, Chief Scientific Officer and co-founder of REVEAL GENOMICS. “This test is demonstrating clinical value in real-world practice across continents and care settings, assisting in the tailoring of chemotherapy intensity in the neoadjuvant setting and supporting chemotherapy-free decisions in the adjuvant setting.”
Patricia Villagrasa, CEO and co-founder, added, “HER2DX was developed with HER2+ disease at its core, and these four high-quality, independent studies reinforce its clinical value. As we continue to grow globally, this is a clear example of how REVEAL GENOMICS is turning cutting-edge science into meaningful solutions that advance personalized cancer care worldwide.”
About HER2DX
HER2DX is the world’s first diagnostic test formulated specifically for HER2+ breast cancer. Marketed by REVEAL GENOMICS since January 2022, the HER2DX is a standardized 27-gene expression test for patients with early-stage HER2+ breast cancer.
HER2DX is a prognostic and predictive assay based on clinical and genomic data. The test integrates clinical information (i.e., tumor size and nodal status) with biological information tracking immune response, luminal differentiation, tumor cell proliferation, and expression of the HER2 17q12-21 chromosomal amplicon, including the ERBB2 gene.
HER2DX®️predicts:
- Risk of relapse score (high vs. low): the risk of recurrence in patients with newly diagnosed HER2+ breast cancer.
- pCR likelihood score (high vs. medium vs. low): the likelihood of a patient responding to anti-HER2-based treatment before surgery.
- ERBB2 score (high vs. medium vs. low): the quantitative expression of ERBB2 mRNA across HER2-negative, HER2-low and HER2+ breast cancer.
About REVEAL GENOMICS
REVEAL GENOMICS, S.L., together with its U.S. subsidiary REVEAL GENOMICS, Inc., is a biotechnology company dedicated to redefining the role of biomarkers in oncology. The company focuses on developing innovative diagnostic tools that optimize therapeutic decision-making for individuals with cancer. By leveraging advanced genomic technologies, sophisticated computational algorithms, and machine learning, REVEAL GENOMICS generates novel insights into cancer biology and treatment response. academic and clinical institutions to advance precision oncology.
REVEAL GENOMICS, S.L. is a spin-off company of Hospital Clínic of Barcelona, IDIBAPS, the University of Barcelona (U.B.), and the Vall d’Hebron Institute of Oncology (VHIO).
REVEAL GENOMICS® and HER2DX® are registered trademarks of REVEAL GENOMICS, S.L.
For further information visit: http://www.reveal-genomics.com
View source version on businesswire.com: https://www.businesswire.com/news/home/20250527779428/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Duravant Completes Acquisition of Pattyn28.5.2025 19:44:00 CEST | Press release
Automation Equipment Leader Duravant expands Packaging Group with Acquisition of Pattyn Duravant LLC (“Duravant”), a global engineered equipment and automation solutions provider to the protein processing, food sorting and handling, packaging, and material handling sectors, announced the completion of its acquisition of the Pattyn Group BV (“Pattyn”), a leading manufacturer of packaging automation systems headquartered in Bruges, Belgium. The transaction was first announced on February 7, 2025, and was completed today. With over 70 years of expertise, Pattyn is a trusted partner for companies looking to automate bulk packaging operations in industries ranging from food and ingredients to non-food applications. Pattyn has been a world-renowned brand specializing in bag-in-box packaging technology, and their comprehensive end-of-line solutions span the entire packaging process from case erecting and bag inserting to weigh filling, bag and case closing, and palletizing. “We are extremely
Rimini Street Appoints Vijay Kumar as EVP and Chief Operating Officer28.5.2025 18:00:00 CEST | Press release
Seasoned business and IT executive leader to drive operational efficiency, scaling, increased profitability and accelerated growth of the business Rimini Street, Inc. (Nasdaq: RMNI), a global provider of end-to-end enterprise software support, management and innovation solutions, and the leading third-party support provider for Oracle, SAP and VMware software, today announced the appointment of Vijay Kumar as executive vice president (EVP) and chief operating officer (COO). This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250528704180/en/ Rimini Street Appoints Vijay Kumar as EVP and Chief Operating Officer In this role, Mr. Kumar leads product management, service strategy and offerings for Rimini Street’s “Support, Optimize and Innovate” solutions and oversees Rimini Street’s Global Capability Centers (GCC). Mr. Kumar also drives operational efficiency and innovation of the portfolio to increase profitability and accelerate t
Cargill Earns #1 Global Ranking for Removing Trans Fats From Edible Oils Portfolio28.5.2025 17:12:00 CEST | Press release
International nutrition foundation recognizes Cargill’s global leadership on industrially produced trans-fatty acid removal Cargill earned the top spot on the inaugural Edible Oil Supplier Index 2025, published by global nonprofit Access to Nutrition initiative (ATNi). The ranking recognizes the company’s leadership in removing industrially produced trans-fatty acids* (iTFAs) from its entire edible oils portfolio, even in markets with no regulatory mandate. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250528074187/en/ Cargill earned the top spot on the inaugural Edible Oil Supplier Index 2025, published by global nonprofit Access to Nutrition initiative (ATNi) Cargill’s leadership in this space has made a tangible difference for customers and consumers around the world. Chantilly, a cherished brand in Mexico known for its bakery, pastry and confectionery products, is a standout example. The company credits Cargill as a key
ARKANCE and Eagle Point Software Extend Partnership to EMEA28.5.2025 15:41:00 CEST | Press release
ARKANCE is now the largest global reseller of Eagle Point’s Learning Management Systems in the AEC and manufacturing sectors ARKANCE, a global leader in digital transformation solutions for the architecture, engineering, and construction (AEC) and manufacturing industries, and Eagle Point Software are extending their partnership to the EMEA region. Building on years of collaboration across the United States and Asia-Pacific, the strengthened global alliance now also delivers next-generation e-learning and digital training solutions to customers across Europe. As AEC firms work to close skills gaps and keep pace with digital transformation, attracting and enabling the next generation of talent is essential. Studies show that 65% of Gen Z say they prefer online learning over in-person classrooms1, making on-demand, cloud-based learning more attractive to new professionals. This makes on-demand, cloud-based learning not just attractive, but urgent. With the extension of the partnership to
Alliance of Four Major Qatari Companies Signs Strategic Agreement with Chinese Automotive Giant Chery28.5.2025 15:15:00 CEST | Press release
A powerful alliance of four leading Qatari companies has signed a strategic agreement with Chery Automobile Co., Ltd., one of China's largest and most innovative automotive manufacturers, to expand Chery’s presence in the global automotive market through a new Qatari-led platform. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250528176705/en/ Alliance of Four Major Qatari Companies Signs Strategic Agreement with Chinese Automotive Giant Chery (Photo: AETOSWire) The alliance includes: Power International Holding Dishley Holding Elite Motors Limited Elaf Motors Chery Automobile was represented at the signing by Mr. Tim Zhang, General Manager – Middle East. The agreement was signed in the presence of senior leadership from both sides. Mr. Mohamad Al Khayyat, representative of the Qatari alliance, stated: "This alliance represents one of the most important partnerships in the regional automotive sector. Through our collaboratio
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom