Magstim EGI
Magstim Granted EU MDR Certification
Magstim Granted EU MDR Certification
Magstim Passes Compliance with Strict European Union Medical Device Regulations
Expanding Magstim Access to Entire EU Marketplace
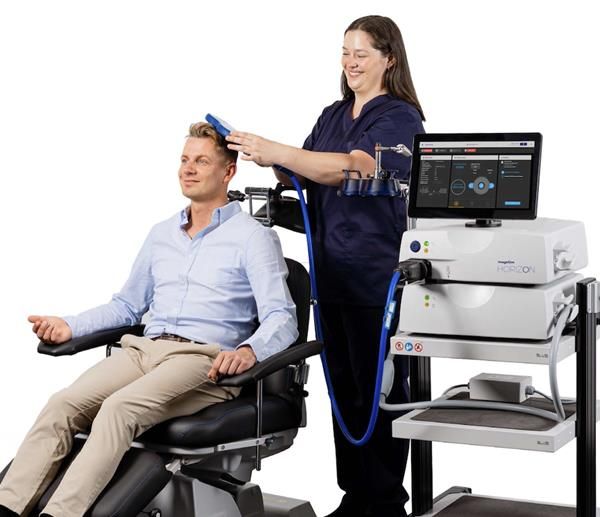
WHITLAND, United Kingdom, May 16, 2025 (GLOBE NEWSWIRE) -- Magstim has been granted EU MDR Certification for all transcranial magnetic stimulation (TMS) systems, demonstrating compliance with strict European Union Medical Device Regulations. This provides a path for Magstim TMS technology to be sold throughout the EU marketplace, and proves that the device is safe, clinically effective and has passed all requirements for testing and traceability.
“The EU MDR is the most rigorous certification backed by verified scientific knowledge,” said Ronnie Stolec-Campo, CEO, Welcony. “As pioneers of neuromodulation for researchers and clinicians, our team is honoured to build world-class technology to study and treat the brain. This certification allows expanded reach to support this life-saving work.”
The European Union Medical Device Regulation (EU MDR) governs the safety, performance, and quality of medical devices sold in the European Economic Area (EEA). It replaced the previous Medical Device Directive (MDD). The certification is issued by a Notified Body, an independent organization designated by an EU member state.
“Our customers can be confident that with this certification, Magstim technology has passed some of the most rigorous quality testing medical devices are subjected to,” said Stolec-Campo. “The big thank you goes to our internal Welcony teams in Regulatory, R&D and support for your commitment to get this across the line—we know this will help people worldwide.”
As the only integrated TMS technology, Magstim is now the only TMS system which can treat MDD, Anxious Depression, OCD, and Adolescent Depression with a single treatment coil. The FDA recently cleared the Magstim Horizon 3.0 and Inspire Transcranial Magnetic Stimulation Systems for treating adolescent patients aged 15-21 for MDD, providing a non-invasive, non-pharmacological technology to improve care.
To learn more about the entire family of research and clinical neurotechnology innovations, visit Magstim.com or call 844-624-7846.
About Welcony
Globally, Welcony technologies have supported thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Interoperative Nerve Monitoring. Welcony is backed by Telegraph Hill Partners, a San Francisco based private equity company.
Media Contact: Mark Sejvar, msejvar@welcony.com, 323-363-3530
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6739d937-ac3a-4dc8-b8a8-f6e50bf48800
Subscribe to releases from Globenewswire
Subscribe to all the latest releases from Globenewswire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Globenewswire
Financière de Tubize SA2.8.2025 08:00:00 CEST | Press release
Financière de Tubize - 2025 half-year financial report
DBV Technologies S.A.1.8.2025 22:05:00 CEST | Press release
Information regarding the total number of voting rights and total number of shares of the Company as of July 31, 2025
Mandalay Resources Corporation1.8.2025 21:12:01 CEST | Press release
Mandalay Obtains Final Order Approving Arrangement with Alkane
F. Hoffmann-La Roche Ltd1.8.2025 20:00:00 CEST | Press release
Roche’s Susvimo maintains vision over five years with two refills per year in people with neovascular age-related macular degeneration (nAMD)
KBC Groep1.8.2025 18:10:00 CEST | Press release
KBC Group: KBC remains well-capitalised under 2025 EU-wide EBA stress test
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom