Codex Labs Corporation
Codex Labs Bia Ultra-Hydrating Soothing Lotion Is Awarded Seal of Acceptance from National Eczema Association and Microbiome-Friendly Certification from MyMicrobiome
Codex Labs Bia Ultra-Hydrating Soothing Lotion Is Awarded Seal of Acceptance from National Eczema Association and Microbiome-Friendly Certification from MyMicrobiome
San Jose, CA, USA, April 23, 2025 (GLOBE NEWSWIRE) -- Codex Labs, a Silicon Valley skintech company, announced that its Bia Ultra-Hydrating Soothing Lotion, the European version of its US Bia over-the-counter (OTC) Bia Eczema Relief Lotion has been awarded the Seal of Acceptance by the National Eczema Association (NEA) in the USA. The NEA Seal of Acceptance™ highlights products that avoid ingredients known to irritate and that are suitable for care of eczema or sensitive skin. The Bia Ultra-Hydrating Soothing Lotion has also achieved Microbiome-friendly certification, according to the MyMicrobiome Standard.
"We are thrilled that our products have been recognized as non-irritating for over 94 million Europeans who complain of uncomfortable skin sensations like itch, burning, or dryness, as well as microbiome-safe. We remain dedicated to creating solutions that protect and restore the skin barrier while supporting the microbiome," says Dr. Barbara Paldus, Founder and CEO.
The Bia Ultra-Hydrating Soothing lotion leverages the patent-pending BiaComplex 2.0™, a blend of biotech-made cellular extracts derived from terrestrial and marine plants. This novel combination of actives addresses hydration, skin barrier repair (both increased ceramide and structural protein production) and itch reduction in distinct yet synergistically-effective ways.
The Bia Ultra-Hydrating Soothing Lotion is designed to be used with the Bia Unscented Soap, the first vegan, MyMicrobiome-certified soap that also carries the NEA Seal of Acceptance. This duo can also be combined with the Antü Skin Barrier Support supplement in an inside-out trio that addresses both gut and skin barrier integrity. The supplement provides l-histidine, an amino acid used by the body to produce filaggrin protein and natural moisturization factor to reinforce the skin barrier, as well as a novel antioxidant (M3Plus™) that helps address gut barrier inflammation and permeability.
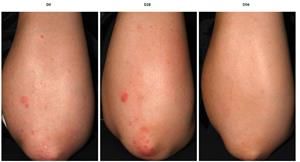
This inside-out trio underwent a clinical trial at Eurofins in Poland on subjects having mild to moderate eczema (SCORAD = 34.5). The measured improvements in 8 weeks included:
- 93% reduction in eczema score (SCORAD) in 100% subjects
- 88% reduction of itch in 97% subjects
- 117% hydration increase in 91% sujects, and
- 83% flakiness (desquamation) decrease in 97% subjects
“Such clinical results demonstrate the importance of addressing skin conditions with both topicals and ingestibles in an integrative, inside-out approach,” stated Dr. Peter Lio, a leading US expert in pediatric eczema and Codex Labs Medical Advisory Board member.
About Codex Labs
Based in Silicon Valley and led by scientist Dr. Barb Paldus, Codex Labs is committed to creating highly effective, clinically proven, microbiome-supporting skin-gut-brain-biome solutions that contain potent, biotech-derived plant-based actives. Our products are focused on restoring/protecting the skin barrier, managing inflammation, and addressing skin conditions associated with aging, acne, eczema and psoriasis. The brand has been heralded by integrative dermatologists and naturopathic doctors for creating the next generation of effective, vegan, cruelty-free and sustainable dermaceutical solutions.
Attachment
Barbara Paldus Codex Labs Corporation (650)4004798 barbara.paldus@codexlabscorp.com
Subscribe to releases from Globenewswire
Subscribe to all the latest releases from Globenewswire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Globenewswire
MoonLake Immunotherapeutics AG25.4.2025 22:05:00 CEST | Press release
MoonLake Immunotherapeutics to host a Capital Markets Day on Tuesday, April 29
T-knife Therapeutics25.4.2025 19:00:00 CEST | Press release
T-knife Therapeutics Presents Preclinical Data at the American Association for Cancer Research (AACR) Annual Meeting Demonstrating a Potential Best-in-Class PRAME Targeted TCR-T Therapy
Zymeworks Inc.25.4.2025 19:00:00 CEST | Press release
Zymeworks Presents New Data from Multiple Development Programs at 2025 AACR Annual Meeting
Sequana Medical NV25.4.2025 18:00:00 CEST | Press release
Press release: Transparency Notification from Shareholders
Sanoma Corp25.4.2025 17:30:00 CEST | Press release
SANOMA CORPORATION: ACQUISITION OF OWN SHARES 25 April 2025
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom