Estithmar Holding Reports an Exceptional 50% Surge in Net Profit to QAR 170 Million
Announcing Q1 2025 Financial Results
Estithmar Holding Q.P.S.C. announced a net profit of QAR 170 million for Q1 2025, reflecting a 50% increase compared to the same period last year. The company highlighted a 64% surge in revenue, reaching QAR 1.3 billion compared to QAR 797 million in Q1 2024. Gross profit rose to QAR 416 million, from QAR 196 million in Q1 2024. EBITDA reached QAR 273 million, marking a 53% increase. Earnings per share also grew by 57%, reaching QAR 0.047.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250421669945/en/
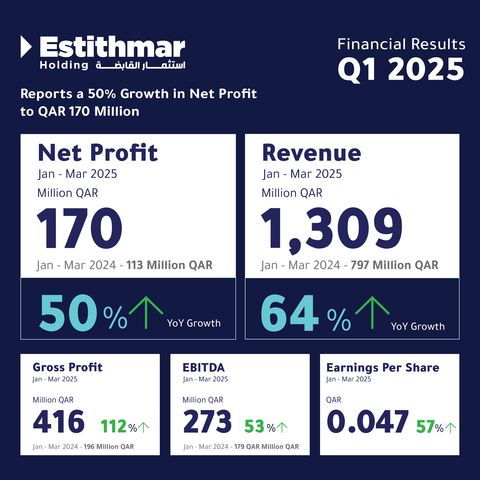
Estithmar Holding Reports an Exceptional 50% Surge in Net Profit to QAR 170 Million (Infographic: AETOSWire)
These strong financial indicators reflect the efficiency of Estithmar Holding’s investment strategy. International projects previously announced by Estithmar Holding started to have a tangible impact on its financial performance in revenue, profits and assets.
The results reflect the achievement of a strategic objective: a balanced contribution to profits and revenues from all four Clusters—Healthcare, Services, Tourism & Real Estate Development, and Contracting & Industries.
The Healthcare cluster posted significant growth in Q1 2025 driven by the cluster’s hospitals outside Qatar which contributed to revenue as new income streams, including Imam Al-Hassan Al-Mujtaba Hospital in Karbala, Al-Nasiriyah Teaching Hospital in Dhi Qar, Iraq, and Misrata Heart & Vascular Center in Libya. Moreover, the growing number of Hospitals outside Qatar in Iraq, Algeria and Libya reflects the confidence that governments across the MENA region have placed in the quality of services provided by Apex Health, the healthcare subsidiary of Estithmar Holding.
The Services cluster maintained leadership in Qatar, especially in Facilities Management and Catering. Expansion into KSA, Jordan and Iraq also significantly contributed to the Cluster’s profitability and the development of new income streams.
The Tourism & Real Estate Development cluster stayed on track with project delivery, including Rixos Baghdad (Iraq) and Rosewood Maldives Resort, driving a QAR 600 million increase in company assets in Q1 2025. Additionally, enhanced efficiency boosted profitability in existing projects, Lusail Winter Wonderland and Al Maha Island.
The Contracting & Industries cluster also made a notable contribution to revenue and profit growth, especially at the peak phase of project deliveries in the Kingdom of Saudi Arabia, including major projects such as the Red Sea Airport and the Yacht Club. The Cluster also secured new projects with Saudi PIF companies and improved local operational efficiency, enhancing profitability in Qatar.
Overall, Estithmar Holding’s Q1 2025 results highlight sustained growth aligned with its strategy to increase shareholder value on the short and the long term.
Group CEO Mr. Juan Leon stated:
“The exceptional rise in all financial indicators reflects the dedication of Estithmar’s team, and I look forward to working closely with them to build on Estithmar Holding’s growth story in Qatar and abroad. Analyzing these results, Estithmar Holding has demonstrated the ability to deliver sustained, diversified growth—both vertically and horizontally—paving the way for further expansion as investor confidence strengthens and our footprint continues to grow both locally and internationally.”
*Source: AETOSWire
View source version on businesswire.com: https://www.businesswire.com/news/home/20250421669945/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Belkin Achieves Qi2.2 Certification for Its Upcoming Products, Unlocking the Future of 25W Wireless Charging15.7.2025 19:06:00 CEST | Press release
With Qi2.2 certification, Belkin reinforces its commitment to quality, safety, and performance for the next generation of wireless charging Belkin, a leading consumer electronics brand for over 40 years, today announced it has received official Qi2.2 certification from the Wireless Power Consortium (WPC) for its upcoming products. As one of the first accessory brands to deliver Qi2.2-certified devices, Belkin is helping bring the next generation of wireless charging to market – enabling faster wireless charging speeds, broader compatibility, and improved performance for consumers. Belkin’s close partnership with the WPC since 2015 has been instrumental in bringing these advancements to consumers. As an early adopter and long-time contributor to WPC standards, Belkin was selected as one of a small group of trusted manufacturers to test and certify Qi2.2 products ahead of the broader industry rollout. All Belkin products undergo rigorous safety, quality, and performance testing. The comp
Cessna Grand Caravan EX to Feature New Executive Interior Options, Expanding Opportunities for Elevated Missions15.7.2025 18:05:00 CEST | Press release
The legendary Cessna Grand Caravan EX will now feature three new executive interior schemes for customers to select when designing their aircraft cabin. The Lunar, Obsidian and Saddle Sport interiors join the existing Canyon and Savanna schemes, providing a broader range of standard choices. The new interior options are available to customers starting this month and allow them to further tailor the interior of their aircraft based on their personal preference or mission. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250715021096/en/ Cessna Grand Caravan EX to feature new executive interior options, expanding opportunities for elevated missions (Photo Credit: Textron Aviation) The Cessna Grand Caravan EX is designed and manufactured by Textron Aviation Inc., a Textron Inc. (NYSE:TXT) company. Premium versions of each of the new interiors are also available, featuring quilted seat stitching and plush carpet, providing an elev
7 Million Tokens Sells Out in less than One Hour—$MBG Token Pre-Sale Shatters Expectations15.7.2025 17:27:00 CEST | Press release
MultiBank Group, the world’s largest and most regulated financial derivatives institution, has set a new benchmark in digital asset launches. The Group’s $MBG Token Pre-Sale sold out in less than one hour with all 7 million tokens fully subscribed across MultiBank.io and Uniswap. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250715055611/en/ The Group’s $MBG Token Pre-Sale sold out in less than one hour with all 7 million tokens fully subscribed across MultiBank.io and Uniswap. Commenting on the success of the Pre-Sale, Naser Taher, Founder and Chairman of MultiBank Group, said: “The sell-out of our initial $MBG Token offering in less than one hour is a decisive validation of our vision. In a market saturated with speculation, the response we received confirms that institutional-grade transparency, regulatory integrity, and asset-backed value are what investors are now demanding. $MBG is here for the long term, reflecting t
First Patient Enrolled in National Cancer Institute’s Vanguard Study Evaluating Guardant Health’s Shield Multi-Cancer Detection Test15.7.2025 17:01:00 CEST | Press release
Study addressing feasibility of using multi-cancer detection tests in future trials aims to enroll up to 24,000 participantsShield MCD reviewed by FDA as part of NCI’s investigational device exemption (IDE) Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced that patient enrollment has begun in the National Cancer Institute (NCI)’s Vanguard Study to evaluate emerging multi-cancer detection (MCD) technology. Guardant’s Shield™ MCD test was selected for use in the four-year study, which aims to enroll up to 24,000 patients and evaluate the use of MCD tests—blood tests that can screen for several types of cancer simultaneously—in future randomized controlled trials. Guardant’s Shield MCD test was chosen for the study based on the overall performance of its Shield platform in detecting 10 cancer types, including lung, breast, colorectal, prostate, bladder, ovarian, pancreatic, esophageal, liver and gastric. The data were presented at the 2025 American
Deltatre Announces Acquisition of Endeavor Streaming to Create Digital and Streaming Platform Leader15.7.2025 16:15:00 CEST | Press release
Deltatre, a leading international provider of streaming, digital, data, and graphics solutions for the sports, media, and entertainment industries, today announced it has entered into a definitive agreement to acquire Endeavor Streaming from Endeavor Group Holdings, Inc. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250715833650/en/ Deltatre announces acquisition of Endeavor Streaming. In bringing together these complementary and proven digital and OTT providers, Deltatre is joining its advanced product suite – D3 VOLT, FORGE, AXIS, and DIVA, which delivers multi-functional digital experiences with integrated video – with Endeavor Streaming’s pure-play OTT product, VESPER. They will also unite their digital strategy, consulting, and direct-to-consumer growth marketing services. The combined business will be best equipped to deliver for sports, media, and entertainment clients through a compelling and comprehensive range of
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom