Decoding Duchenne Muscular Dystrophy: MRI Study Explores the Relationship Between AMRA’s Muscle Biomarkers and Functional Changes in Individuals With DMD
Recent AMRA research examines whether the use of MRI-based biomarkers as surrogate endpoints in DMD clinical trials is feasible, allowing for more informative descriptions of disease severity and progression in DMD
Researchers from AMRA Medical, in collaboration with Pfizer, Inc., have recently published a study demonstrating the utility of AMRA’s muscle biomarkers as surrogate endpoints to describe functional changes in participants with Duchenne Muscular Dystrophy (DMD). The study explored changes in lean muscle volume (LMV), muscle fat fraction (MFF), and muscle fat infiltration (MFI) – as well as their relationship to changes in physical performance as described by the North Star Ambulatory Assessment (NSAA) – in order to determine whether MRI-based muscle biomarkers have the potential to detect and quantify clinically meaningful changes in DMD.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250415308325/en/
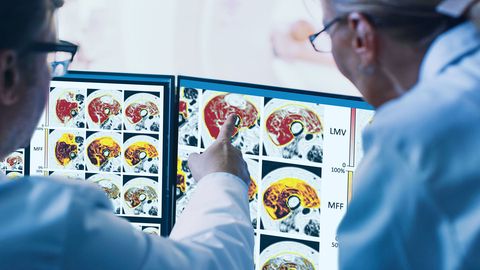
AMRA's muscle biomarkers - Research study explore changes in lean muscle volume (LMV), muscle fat fraction (MFF), and muscle fat infiltration (MFI)
Magnetic resonance imaging (MRI) data from a Phase II clinical trial for domagrozumab in children were re-analyzed using AMRA® Researcher with corresponding functional data at baseline, and at follow-up after 49 and 97 weeks. Findings showed that LMV, MFF, and MFI all correlated with muscle strength and NSAA scores at baseline and at follow-ups, and that equally strong correlations were observed when comparing changes in MRI biomarkers after 49 weeks to changes in NSAA score after 97 weeks.
The study found not only that AMRA’s MRI-based muscle quantification measures are associated with physical function and disease progression in DMD, but also that these measures may be able to augment clinical research studies in DMD by serving as biomarkers of DMD-associated changes prior to clinical manifestations.
Furthermore, the study builds on previous work by AMRA in the neuromuscular disease space, notably in sIBM and FSHD. Our early success in demonstrating the utility of these fat-referenced MRI-based muscle composition biomarkers in clinical research applications of heterogeneous and slowly-progressing muscle dystrophies such as FSHD, now in more rapidly-progressing diseases such as DMD, as well as in inflammatory myopathies such as sIBM, is a testament to the reproducibility of these composite measurements for use as clinical research tools in a number of neuromuscular disease trials.
AMRA is committed to helping pioneer the next wave of neuromuscular disease trials using MRI-based analyses, by delivering biomarkers that enable standardized evaluation of various endpoints in neuromuscular disease research. With AMRA, researchers can augment clinical trials with powerful endpoints to gain more holistic insights and, ultimately, advance their clinical pipeline.
You can read the full publication inMuscle & Nerve, titled “Relationship Between Quantitative Magnetic Resonance Imaging Measures and Functional Changes in Patients With Duchenne Muscular Dystrophy”, here. You can also learn more about how AMRA’s unique MRI-based biomarker methodologies can improve your neuromuscular disease trials here, or by reaching out to info@amramedical.com.
About AMRA Medical
AMRA Medical is a health informatics and precision medicine company that is pioneering body composition analysis, providing cutting-edge solutions to advance both clinical research and patient care initiatives. AMRA's gold-standard technology delivers multiple fat and muscle biomarkers - derived simply from rapid whole-body MRI scans. AMRA is committed to driving transformative care and simplifying vital decision-making in both research and clinical care settings by offering support services via their innovative platform.
Learn more about AMRA Medical’s MRI-based solutions at https://amramedical.com/solutions, or connect with our team of experts for a detailed discussion at info@amramedical.com.
Follow AMRA on LinkedIn for the latest updates in fat distribution and muscle composition assessments in metabolic disease research and beyond.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250415308325/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Kinaxis Launches Tariff Response Solution to Help Supply Chains Adapt to Disruption with Confidence17.4.2025 19:30:00 CEST | Press release
New solution simulates tariff impacts, aligns teams, and enables action in under three weeks As ongoing tariff pressures and trade uncertainty continue to reshape global supply chains, Kinaxis® (TSX:KXS), the leader in real-time supply chain orchestration, today launched Kinaxis Tariff Response - a new offering that helps companies simulate tariff exposure, run strategic scenarios, and make data-informed decisions quickly. Built on the company’s AI-powered Maestro™ platform and delivered by Kinaxis supply chain experts, the service can be live in as few as 21 days, giving planners access to tariff modeling without the cost or complexity of building it internally. The solution meets rising demand for scenario planning - providing a faster, more accessible way for companies to shift from reactive firefighting to proactive orchestration. While AI-powered what-if scenario planning has long been a core capability of Maestro, Kinaxis Tariff Response builds on that foundation with a focused s
Rigaku and SPERA PHARMA Initiate Strategic Partnership to Advanced Pharmaceutical Development17.4.2025 17:00:00 CEST | Press release
Rigaku Corporation, a Group company of Rigaku Holdings Corporation (headquarters: Akishima, Tokyo; CEO: Jun Kawakami; hereinafter “Rigaku”), and SPERA PHARMA, Inc. (headquarters: Osaka; President & Representative Director: Keitaro Iwaki; hereinafter “SPERA PHARMA”) have initiated a strategic partnership aimed at accelerating and transforming pharmaceutical development by leveraging the two companies’ cutting-edge technologies and extensive expertise. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250417047994/en/ Left: Keitaro Iwaki, President & Representative Director, SPERA PHARMA; Right: Jun Kawakami, President & CEO, Rigaku SPERA PHARMA will use XtaLAB Synergy-ED, an integrated platform for electronic diffraction provided by Rigaku, for contract analytical services, thereby offering customers robust support in pharmaceutical development. The XtaLAB Synergy-ED incorporates 3D ED/MicroED1, which is utilized for the structu
CORRECTING and REPLACING MultiSafepay Supporting 20,000 SMEs to Scale Through Payment Integration and Digitisation with Antom Technology Engine17.4.2025 15:36:00 CEST | Press release
MultiSafepay, the Amsterdam-based digital payment provider, now serves 20,000+ SME merchants across Europe, with merchant numbers growing 11% since its strategic integration with Antom, empowering thousands of businesses to thrive. Transaction volumes have surged, growing by 44% YoY, driven organically by rapid deployment of POS solutions and collaboration with Antom. Ant International has opened a new office in Amsterdam’s City Centre for MultiSafepay which enables more efficient collaboration with Ant International’s other business pillars. Seventh paragraph, first sentence should read: Today, 8% of MSP’s total processed transaction volume is handled through in-person payments, using traditional payment terminal technology (such as C-TAP terminals) as well as Smart POS devices which offer merchants digital tools and do more than just accept payments. (instead of MSP’s total processed transaction volume is handled through in-person payments, using traditional payment terminal technolo
MultiSafepay Supporting 20,000 SMEs to Scale Through Payment Integration and Digitisation with Antom Technology Engine17.4.2025 15:36:00 CEST | Press release
MultiSafepay, the Amsterdam-based digital payment provider, now serves 20,000+ SME merchants across Europe, with merchant numbers growing 11% since its strategic integration with Antom, empowering thousands of businesses to thrive. Transaction volumes have surged, growing by 44% YoY, driven organically by rapid deployment of POS solutions and collaboration with Antom. Ant International has opened a new office in Amsterdam’s City Centre for MultiSafepay which enables more efficient collaboration with Ant International’s other business pillars. MultiSafepay (MSP), an Amsterdam-based payment service provider, which became part of Ant International’s Antom, reported strong growth since its strategic integration with Antom, supporting thousands more SMEs in Europe to scale through innovative solutions. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250417858076/en/ Ant International’s new office in Amsterdam City Centre will hous
Avania Strengthens Leadership Team With Pivotal Executive Appointments17.4.2025 15:00:00 CEST | Press release
Avania — the leading global MedTech advisory and clinical development partner — today announced the appointments of Jasmine Saba as sr. vice president of strategic relationships and Josh Blacker as sr. vice president of global business development. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250417247152/en/ Jasmine Saba (left) has been appointed Sr. Vice President of Strategic Relationships. Josh Blacker (right) joins Avania as Sr. Vice President of Global Business Development. Saba Appointed Sr. Vice President of Strategic Relationships Saba will take on this new role to support Avania’s growth by identifying, building, and nurturing key partnerships with global multinational medical technology companies. She will develop repeatable and scalable solutions leveraging Avania’s partnership approach as these customers increasingly rely on Avania to be an extension of their existing teams. With her extensive experience in R&
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom