Looking? Grindr’s ‘Right Now’ Feature Expands into 15 New Cities
More cities, more connections, less waiting – Right Now makes finding a hookup faster than ever
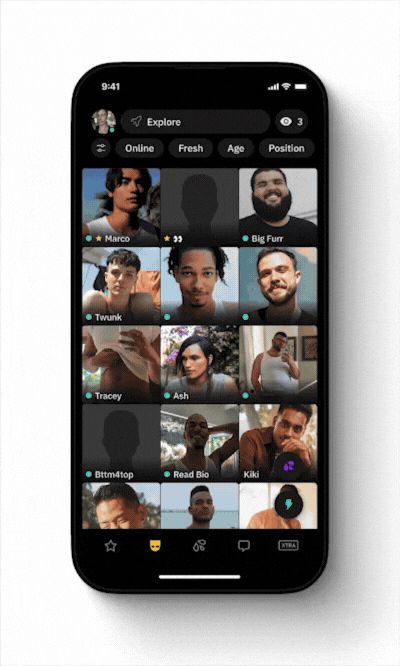
Grindr, the Global Gayborhood in Your Pocket™ with more than 14.5M average monthly active users, today announced the expansion of Right Now, their intent-based feature designed to instantly connect people looking for immediate encounters. Right Now is a real-time feed, separate from the grid, where Grindr users can post text and share photos displayed for one hour that let others know exactly what they’re looking for… right now.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250325291399/en/
Grindr's Right Now feature.
Today, Right Now will begin rolling out in fifteen new cities across North America, Europe, and South America. New York City, Los Angeles, Miami, Philadelphia, Austin, Chicago, Houston, Atlanta, Orlando, London, Paris, Madrid, Milan, Amsterdam, and Sao Paulo - some of the most densely populated gayborhoods in the world.
“Right Now empowers our users to find exactly what they want, when they want it – without the guesswork,” said AJ Balance, Chief Product Officer at Grindr. “We built this intention-based feature based on feedback from our community so they can connect with like-minded people, without wasting time on mismatched expectations. Too often, people start a conversation only to realize they’re looking for different things – one person wants a date, the other a quick connection. Right Now makes it clear who’s available and what they’re looking for, in real time.”
Right Now is accessible on iOS and Android, via the main navigation bar, side bar, and a floating action button on the main grid, leading people to a separate feed optimized for real-time engagement. At launch, people will receive ten complimentary hour-long sessions per week, refreshing every Friday. After using their complimentary sessions, they will have the option to purchase additional Right Now sessions.
This expansion builds on the successful 2024 pilots of Right Now in Australia and the greater Washington D.C. area, reinforcing Grindr’s commitment to creating more opportunities for successful, intention-based connections. Stay tuned for future updates as Grindr expands the feature to more locations around the world.
Explore our product roadmap for a closer look at the new features coming to Grindr in 2025.
About Grindr Inc.
With more than 14.5 million average monthly active users, Grindr (NYSE:GRND) has grown to become the Global Gayborhood in Your Pocket™, on a mission to make a world where the lives of our global community are free, equal, and just. Available in 190 countries and territories, Grindr is often the primary way for our users to connect, express themselves, and discover the world around them. Since 2015, Grindr for Equality has advanced human rights, health, and safety for millions of LGBTQ+ people in partnership with organizations in every region of the world. Grindr has offices in West Hollywood, the Bay Area, Chicago, and New York. The Grindr app is available on the App Store and Google Play.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250325291399/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
4Moving Biotech Enrolls First Patient in Phase 2a Trial of 4P004, a Potential First-in-Class GLP-1 Therapy for Knee Osteoarthritis16.7.2025 07:00:00 CEST | Press release
- First patient enrolled in INFLAM MOTION, a global randomized Phase 2a trial including 129 knee osteoarthritis patients - 4P004 to be evaluated over 3 months for dual efficacy: symptom relief and synovial health improvement via contrast-enhanced MRI - Topline results expected in the second half of 2026 4Moving Biotech (4MB), a spin-off of 4P-Pharma dedicated to developing first-in-class treatments that modify the natural course of knee osteoarthritis (OA), today announced that the first patient has been enrolled in Phase 2a clinical trial, INFLAM MOTION. The study will evaluate 4P004, an intra-articular GLP-1 analog, as a potential first-in-class therapeutic candidate for knee osteoarthritis. INFLAM MOTION is a multicenter, randomized, double-blind, placebo-controlled Phase 2a trial planned to be conducted across Europe, the United States, and Canada. A total of 129 patients worldwide diagnosed with knee OA will be enrolled to evaluate, for the first time in humans, the efficacy of 4P
Belkin Achieves Qi2.2 Certification for Its Upcoming Products, Unlocking the Future of 25W Wireless Charging15.7.2025 19:06:00 CEST | Press release
With Qi2.2 certification, Belkin reinforces its commitment to quality, safety, and performance for the next generation of wireless charging Belkin, a leading consumer electronics brand for over 40 years, today announced it has received official Qi2.2 certification from the Wireless Power Consortium (WPC) for its upcoming products. As one of the first accessory brands to deliver Qi2.2-certified devices, Belkin is helping bring the next generation of wireless charging to market – enabling faster wireless charging speeds, broader compatibility, and improved performance for consumers. Belkin’s close partnership with the WPC since 2015 has been instrumental in bringing these advancements to consumers. As an early adopter and long-time contributor to WPC standards, Belkin was selected as one of a small group of trusted manufacturers to test and certify Qi2.2 products ahead of the broader industry rollout. All Belkin products undergo rigorous safety, quality, and performance testing. The comp
Cessna Grand Caravan EX to Feature New Executive Interior Options, Expanding Opportunities for Elevated Missions15.7.2025 18:05:00 CEST | Press release
The legendary Cessna Grand Caravan EX will now feature three new executive interior schemes for customers to select when designing their aircraft cabin. The Lunar, Obsidian and Saddle Sport interiors join the existing Canyon and Savanna schemes, providing a broader range of standard choices. The new interior options are available to customers starting this month and allow them to further tailor the interior of their aircraft based on their personal preference or mission. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250715021096/en/ Cessna Grand Caravan EX to feature new executive interior options, expanding opportunities for elevated missions (Photo Credit: Textron Aviation) The Cessna Grand Caravan EX is designed and manufactured by Textron Aviation Inc., a Textron Inc. (NYSE:TXT) company. Premium versions of each of the new interiors are also available, featuring quilted seat stitching and plush carpet, providing an elev
7 Million Tokens Sells Out in less than One Hour—$MBG Token Pre-Sale Shatters Expectations15.7.2025 17:27:00 CEST | Press release
MultiBank Group, the world’s largest and most regulated financial derivatives institution, has set a new benchmark in digital asset launches. The Group’s $MBG Token Pre-Sale sold out in less than one hour with all 7 million tokens fully subscribed across MultiBank.io and Uniswap. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250715055611/en/ The Group’s $MBG Token Pre-Sale sold out in less than one hour with all 7 million tokens fully subscribed across MultiBank.io and Uniswap. Commenting on the success of the Pre-Sale, Naser Taher, Founder and Chairman of MultiBank Group, said: “The sell-out of our initial $MBG Token offering in less than one hour is a decisive validation of our vision. In a market saturated with speculation, the response we received confirms that institutional-grade transparency, regulatory integrity, and asset-backed value are what investors are now demanding. $MBG is here for the long term, reflecting t
First Patient Enrolled in National Cancer Institute’s Vanguard Study Evaluating Guardant Health’s Shield Multi-Cancer Detection Test15.7.2025 17:01:00 CEST | Press release
Study addressing feasibility of using multi-cancer detection tests in future trials aims to enroll up to 24,000 participantsShield MCD reviewed by FDA as part of NCI’s investigational device exemption (IDE) Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced that patient enrollment has begun in the National Cancer Institute (NCI)’s Vanguard Study to evaluate emerging multi-cancer detection (MCD) technology. Guardant’s Shield™ MCD test was selected for use in the four-year study, which aims to enroll up to 24,000 patients and evaluate the use of MCD tests—blood tests that can screen for several types of cancer simultaneously—in future randomized controlled trials. Guardant’s Shield MCD test was chosen for the study based on the overall performance of its Shield platform in detecting 10 cancer types, including lung, breast, colorectal, prostate, bladder, ovarian, pancreatic, esophageal, liver and gastric. The data were presented at the 2025 American
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom