Koelis
Koelis Announces Innovative Products at EAU 2025 enhancing MRI Fusion Biopsy Workflows and promoting Focal Therapy in Prostate Cancer
Koelis Announces Innovative Products at EAU 2025 enhancing MRI Fusion Biopsy Workflows and promoting Focal Therapy in Prostate Cancer
GRENOBLE, France, March 20, 2025 (GLOBE NEWSWIRE) -- Koelis, SAS (“Koelis” or the “Company”, www.koelis.com), a global leader and innovator in urology and prostate care, announced today it is attending its 17th European Urology Meeting EAU as a major sponsor and exhibitor.
Koelis will be holding interactive demonstrations at its booth #C40 from March 21st to March 24th to promote the innovative approach of using its exclusive “Organ Based Tracking” fusion technology in transperineal focal therapy of prostate cancer.
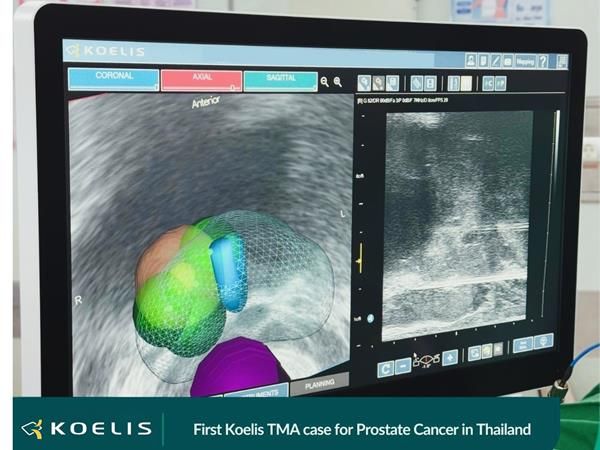
Fusion biopsy and patient follow-up with 3D mapping are the cornerstone of a successful Focal Therapy program. In line with the latest EAU guidelines, Koelis Trinity® is a unique device on the market integrating technologies to ensure continuity between fusion biopsy and focal guidance.
Trinity integrates 3D ultrasound imaging with proprietary MRI-US fusion image guidance that features the Company’s unique prostate motion tracking software (OBT Fusion®). The compact Koelis Trinity® system does not require interface with either external ultrasound equipment or external sensors. Its versatility is enabling Koelis to lead the ongoing paradigm shift in prostate cancer care towards more accurate biopsy diagnoses, personalized patient follow-up and more choices for less invasive, ambulatory treatments.
During EAU 2025, Koelis will demonstrate its capacity to plan and guide Focal Therapy.
Dr Jonathan Fainberg of Memorial Sloan Kettering, NY USA, will introduce an innovative way of managing focal therapy for radio-recurrent prostate cancer using the full capacity of the Koelis Trinity®. His video abstract entitled “Focal salvage cryoablation using organ-based tracking and software-guided ‘triple fusion’” will be presented during the Sunday Video Session 14 at 17:15.
Dr Peter Chiu of Prince of Wales Hospital, Hong Kong, will chair the abstract session 36 on Sunday 17:15 entitled “New technologies and focal therapy in localised prostate cancer”. He will also present in abstract session 43, Monday 10:45, “Navigating prostate biopsy strategies: MRI-targeted, systematic, and perilesional approaches”, which is essential for focal therapy preparation.
Koelis will more specifically display at its booth its own focal therapy offering: Targeted Microwave Ablation (“TMA”) performed transperineally with Trinity guidance which offers a very simple, fast, precise, efficient and safe outpatient focal procedure.
Supporting its focal therapy capacity, Koelis will also be featuring its “Promap-Contour” software which enables healthcare providers to utilize their compatible AI/connectivity solution to automatically process prostate contours and lesions. MRI preparation outputs are then seamlessly transferred to the KOELIS Trinity® platform for 3D MRI/US fusion procedures. In this regard, Koelis has entered into collaboration with respected companies from the AI/connectivity field: Siemens Healthineers, Deephealth, Quibim, Bot Image, and more to come.
Antoine Leroy, PhD, Koelis founder and CEO, declared: "In 2025, Koelis emphasizes workflow efficiency and focal therapy. We are proud to respond to the growing demand and to the latest guidelines by innovating and meeting the needs of urologists and their patients. We have the best-in-class fusion technology on the market, and our latest announcements made during EAU’25 will support our customers innovating efficiently.”
KOELIS technology is currently available in 50 countries with over 600 systems in use in the USA, Europe and Asia. The Koelis Trinity® platform has been utilized in 1 million patients diagnosed with prostate cancer.
About KOELIS
Headquartered in Grenoble, France, Koelis has been a pioneer and leader of MRI-US fusion image guidance technology since 2006. Featuring proprietary 3D ultrasound and prostate motion tracking software (OBT Fusion®), the Koelis Trinity® system facilitates more accurate biopsy diagnosis as well as enabling “focal” prostate cancer treatment alternatives to traditional “total” organ treatments such as surgical prostatectomy and radiation. The Company’s commitment to minimally invasive prostate cancer treatment includes multi-center clinical registries (“Violette” in Europe, NCT04582656, and “Violetta” in Asia, NCT06262633) in Europe based on Trinity-guided microwave ablation technology. Koelis has offices in Grenoble (France), Princeton (NJ, USA), Saarbrücken (Germany) and Singapore to serve more than 50 countries. Learn more about KOELIS at www.koelis.com.
Contact press :
Thomas MARTINS PINHEIRO
Communication Manager
Tél. : + 33 (0)7 88 31 16 48
Thomas.martins-pinheiro@koelis.com
A photo accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/2926aae4-e94f-4316-8160-c09b41d8f41e
Subscribe to releases from Globenewswire
Subscribe to all the latest releases from Globenewswire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Globenewswire
Golden Ocean Group Limited20.3.2025 23:00:00 CET | Press release
GOGL - Filing of 2024 Annual Report on Form 20-F
Sportradar AG20.3.2025 21:49:09 CET | Press release
Sportradar Files Its Annual Report on Form 20-F
Nokia Oyj20.3.2025 21:30:00 CET | Press release
Nokia Corporation: Repurchase of own shares on 20.03.2025
SCOR20.3.2025 19:26:35 CET | Press release
SCOR SE announces the availability of its 2024 Universal Registration Document
Huhtamäki Oyj20.3.2025 19:00:00 CET | Press release
Huhtamäki Oyj - Managers' Transactions (O'Hara)
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom