RevBio Initiates its Pivotal Clinical Trial in Europe for its Dental Implant Stabilization Product
17.3.2025 20:28:00 CET | Business Wire | Press release
The Company Received Approval in Multiple European Countries to Initiate this Key Study Necessary for Commercial Product Approval
RevBio, Inc., announced that it has received regulatory and ethics committee approvals in multiple European countries to conduct its pivotal clinical trial for its dental implant stabilization product. The successful completion of this pivotal clinical trial will result in the CE marking approval for the product, which will allow the company to begin commercial sales in Europe. As of the date of this press release, the company has already enrolled 30 of an expected 75 patients in this clinical trial.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250306978603/en/

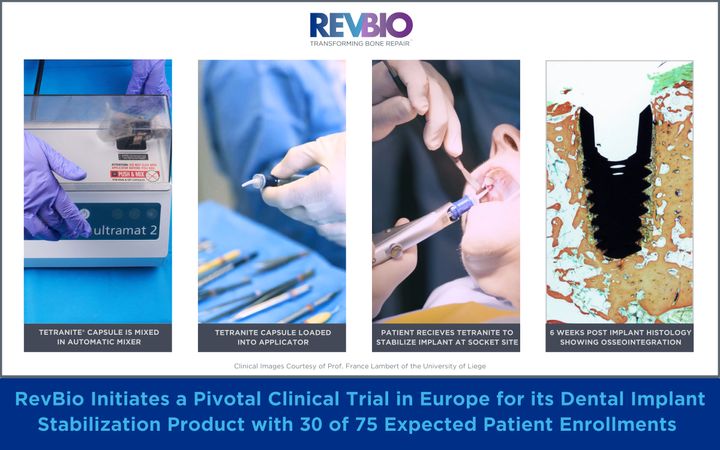
RevBio initiates a pivotal clinical trial in Europe for its Dental Implant Stabilization product with 30 of 75 expected patient enrollments. Clinical Images courtesy of Prof. France Lambert of the University of Liege.
“The results from this ongoing international multicenter study are exceeding expectations, showcasing remarkable outcomes. We are profoundly optimistic and incredibly enthusiastic about what the future holds,” said Prof. Dr. med. Dent., Patrick Schmidlin, head of the Division of Periodontology at the University of Zurich, who serves as one of the investigators in RevBio’s pivotal clinical trial. “This breakthrough material promises to open a new chapter in dental care, redefining what is possible in regenerative dentistry.”
RevBio received regulatory and ethics committee approvals for five clinical sites consisting of one in Switzerland, two in Belgium, one in Spain, and one in the United Kingdom. Each site is expected to enroll between 12-25 patients for a total of approximately 75 patients. The investigators who are approved to enroll patients in this clinical trial are Prof. Dr. med. Dent., PhD, Patrick Schmidlin, head of the Department of Periodontology at the University of Zurich, Ana Castro, BDS MSc PhD, Clinical Head Department Periodontology at the Catholic University of Leuven, France Lambert, DDS, PhD, Professor and Head of Periodontology, Oral Surgery, and Implant Surgery at the University of Liege, Arturo Llobell, DDS, MS, a periodontist in private practice in Valencia, Spain, and Azim Malik, BDS, MFDS RCSEd, DipPCD RCSI, DClinDent, MPerio RCSEd, a periodontist and implant specialist in private practice in London, United Kingdom.
When teeth are extracted due to damage from traumatic injuries, tooth decay, or gum disease, the current standard of care consists of multiple staged surgical procedures to restore a patient’s dentition with prosthetic crowns supported by dental implants. Frequently, extraction sites are too large for dental implants to achieve primary stability through conventional mechanical engagement. Instead, patients must undergo a costly, complex, and lengthy process including a preliminary bone grafting surgery before receiving a dental implant. The use of TETRANITE to stabilize an unstable implant will allow for the immediate placement of dental implants which otherwise could not be placed until the initial bone graft has healed to form new bone. As a result, the TETRANITE® biomaterial will help reduce the duration and complexity of these dental implant procedures, lessen patient pain and recovery time, and reduce the overall cost of care thereby providing greater patient access for the treatment of tooth loss.
"The approval to conduct this pivotal clinical trial and the successful enrollment of the first 30 patients is a watershed moment for RevBio," said Alan Pollack, DDS, RevBio’s Senior Director of Dental Clinical Operations. “This clinical trial has the potential to significantly accelerate the timeframe when this promising bone adhesive technology can be used in the field of dentistry.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of TETRANITE®, a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's TETRANITE technology is not yet approved for commercial use.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250306978603/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
LZE GmbH Introduces Fraunhofer’s RFicient® Technology to the Market12.3.2026 14:51:00 CET | Press release
LZE GmbH is expanding its technology transfer portfolio and making the RFicient® ultra-low-power wake-up receiver technology from the Fraunhofer Institute for Integrated Circuits IIS available for the first time as a standard chip for close-to-production industrial applications. The solution enables energy-efficient IoT designs that remain continuously reachable while consuming only microamps – a key step for long-lasting, low-maintenance IoT products. LZE GmbH drives technology transfer to market: standard chip availability for close-to-production applications As a bridge between research and industry, LZE GmbH is making it easier for companies to access innovative technologies and helping them to quickly and reliably transform new developments into market-ready solutions. With RFicient®-IC (FH101RF), LZE is providing another high-tech product that comes directly from Fraunhofer research and can now be ordered in volume and integrated into close-to-production product development for t
Owkin Creates New Spin out Waiv, Formerly Owkin Dx, With $33M Financing12.3.2026 14:30:00 CET | Press release
Investment lead by OTB Ventures and Alpha Intelligence CapitalWaiv develops AI-powered precision testing to better identify and stratify patients in the clinic and in clinical trials, transforming patient careWaiv extends Owkin’s strategy of real-world validation for its AI Owkin, the AI company on a mission to solve the complexity of biology, today announced the spin out of Waiv, formerly known as Owkin Dx. The move follows significant investor interest and positions Waiv to bring AI-powered precision testing for better identification of patients in the clinic and in clinical trials, to transform patient care. This follows on from the successful launch of Bioptimus, an Owkin incubated company, in February 2024. Waiv translates AI innovation into real-world clinical impact, developing tests that predict biomarkers and patient outcomes, including RlapsRisk BC for prognostic risk profiling. With multiple tests already in use in clinical settings, its deployment platform Destra, and colla
RQM+ Launches SMART Solutions Life Cycle Partnership Model12.3.2026 14:30:00 CET | Press release
Helps MedTech Companies Navigate MDR, IVDR, and AI-Enabled Device Development RQM+, a leading MedTech CRO offering regulatory consulting, clinical trial, laboratory, and reimbursement services, today announced the launch of SMART Solutions, a life cycle partnership model designed to help medical device and diagnostics companies manage growing regulatory and development complexity. SMART Solutions introduces a strategy-led operating framework that unifies regulatory, quality, clinical, reimbursement, and laboratory expertise to support MedTech companies across the entire product life cycle to help reduce risk from early development through post-market. “MedTech companies are navigating unprecedented complexity as regulatory expectations evolve, product innovation accelerates, and post-market expectations are expanding,” said John Potthoff, Ph.D., chief executive officer of RQM+. “SMART Solutions moves beyond traditional consulting by providing an integrated life cycle partnership that h
Cryptio Raises $45m Series B as Digital Assets Move Into Regulated Financial Markets12.3.2026 14:06:00 CET | Press release
The system of record for tokenized finance – ERP infrastructure for institutions operating in digital assets Cryptio, a leader in financial data transformation and enterprise resource planning (ERP) applications for regulated digital assets, announced today a $45 million Series B funding round co-led by BlackFin Capital Partners and Sentinel Global, with participation from 1kx, Alven, BlueYard Capital and Ledger Cathay Capital. Banks, exchanges, asset managers, including Société Générale’s SG Forge, Circle, Gemini, and Securitize rely on Cryptio to ensure financial integrity across their digital assets businesses. Existing ERP systems fall short for digital assets Traditional ERP and accounting systems were not designed for blockchain-native assets, real-time reporting, or modern custody frameworks. As regulated financial institutions expand into stablecoins, tokenized securities and other on-chain instruments, these limitations create material operational and reporting challenges. Cry
HyperLight Introduces 145 GHz Reference Modulators to Enable 448Gbps per Lane Datacom and 260GBaud Telecom Development12.3.2026 14:03:00 CET | Press release
HyperLight Corporation, creator of the TFLN Chiplet™ platform, today announced the release of its 145 GHz Packaged Intensity Modulator (IM), expanding the company‘s high-speed modulator portfolio. The new device is designed for ultra-wide modulation bandwidth, high signal fidelity, and stable operation control, enabling 448 Gbps per lane intensity-modulated-direct-detection (IMDD), 260 GBaud coherent links, and broadband RF photonics systems. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260312319685/en/ Fig. 1: HyperLight’s 145 GHz intensity modulator for 448Gbps per lane IMDD and 260GBaud coherent applications, with operational electro-optical bandwidth >145GHz, stable bias control, 0.8 mm-connector; available in O-, C-, and L-bands. As symbol rates and analog bandwidth requirements continue to rise across data center interconnects, AI-driven photonics infrastructure, and laboratory test environments, system architects in
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom