RevBio Initiates its Pivotal Clinical Trial in Europe for its Dental Implant Stabilization Product
17.3.2025 20:28:00 CET | Business Wire | Press release
The Company Received Approval in Multiple European Countries to Initiate this Key Study Necessary for Commercial Product Approval
RevBio, Inc., announced that it has received regulatory and ethics committee approvals in multiple European countries to conduct its pivotal clinical trial for its dental implant stabilization product. The successful completion of this pivotal clinical trial will result in the CE marking approval for the product, which will allow the company to begin commercial sales in Europe. As of the date of this press release, the company has already enrolled 30 of an expected 75 patients in this clinical trial.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250306978603/en/

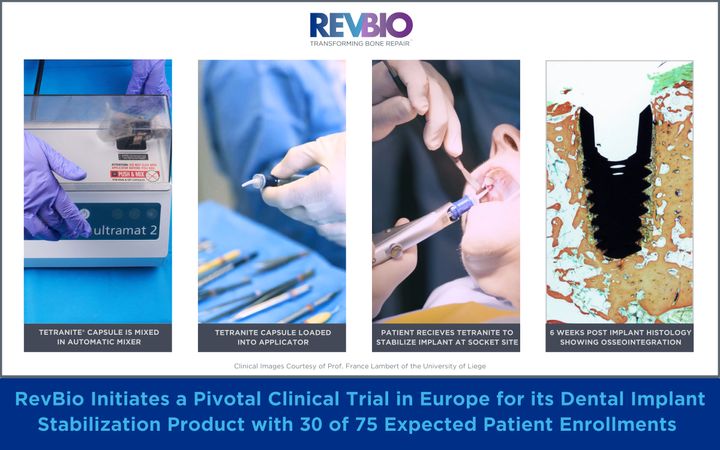
RevBio initiates a pivotal clinical trial in Europe for its Dental Implant Stabilization product with 30 of 75 expected patient enrollments. Clinical Images courtesy of Prof. France Lambert of the University of Liege.
“The results from this ongoing international multicenter study are exceeding expectations, showcasing remarkable outcomes. We are profoundly optimistic and incredibly enthusiastic about what the future holds,” said Prof. Dr. med. Dent., Patrick Schmidlin, head of the Division of Periodontology at the University of Zurich, who serves as one of the investigators in RevBio’s pivotal clinical trial. “This breakthrough material promises to open a new chapter in dental care, redefining what is possible in regenerative dentistry.”
RevBio received regulatory and ethics committee approvals for five clinical sites consisting of one in Switzerland, two in Belgium, one in Spain, and one in the United Kingdom. Each site is expected to enroll between 12-25 patients for a total of approximately 75 patients. The investigators who are approved to enroll patients in this clinical trial are Prof. Dr. med. Dent., PhD, Patrick Schmidlin, head of the Department of Periodontology at the University of Zurich, Ana Castro, BDS MSc PhD, Clinical Head Department Periodontology at the Catholic University of Leuven, France Lambert, DDS, PhD, Professor and Head of Periodontology, Oral Surgery, and Implant Surgery at the University of Liege, Arturo Llobell, DDS, MS, a periodontist in private practice in Valencia, Spain, and Azim Malik, BDS, MFDS RCSEd, DipPCD RCSI, DClinDent, MPerio RCSEd, a periodontist and implant specialist in private practice in London, United Kingdom.
When teeth are extracted due to damage from traumatic injuries, tooth decay, or gum disease, the current standard of care consists of multiple staged surgical procedures to restore a patient’s dentition with prosthetic crowns supported by dental implants. Frequently, extraction sites are too large for dental implants to achieve primary stability through conventional mechanical engagement. Instead, patients must undergo a costly, complex, and lengthy process including a preliminary bone grafting surgery before receiving a dental implant. The use of TETRANITE to stabilize an unstable implant will allow for the immediate placement of dental implants which otherwise could not be placed until the initial bone graft has healed to form new bone. As a result, the TETRANITE® biomaterial will help reduce the duration and complexity of these dental implant procedures, lessen patient pain and recovery time, and reduce the overall cost of care thereby providing greater patient access for the treatment of tooth loss.
"The approval to conduct this pivotal clinical trial and the successful enrollment of the first 30 patients is a watershed moment for RevBio," said Alan Pollack, DDS, RevBio’s Senior Director of Dental Clinical Operations. “This clinical trial has the potential to significantly accelerate the timeframe when this promising bone adhesive technology can be used in the field of dentistry.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of TETRANITE®, a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's TETRANITE technology is not yet approved for commercial use.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250306978603/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
AI Meets Traditional Culture: Huangshan Captures Widespread Attention at ITB Berlin7.3.2026 10:22:00 CET | Press release
Huangshan, one of China’s most iconic scenic destinations, drew significant attention at this year’s ITB by presenting a compelling fusion of traditional Chinese culture and cutting-edge artificial intelligence under the slogan “The world of Huangshan is for the world.” This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260307909978/en/ International visitor admires Huangshan cultural and creative exhibits at the Huangshan stand during ITB Berlin. Located in eastern China’s Anhui Province, Huangshan is famed for its “Five Natural Wonders” — fantastic pines, grotesque rocks, sea of clouds, hot spring and winter snow. The mountain is widely regarded as one of China’s greatest mountain landscapes. It is also a rare natural heritage site that simultaneously holds multiple international designations, including UNESCO World Cultural and Natural Heritage status, a UNESCO Global Geopark and a World Biosphere Reserve. At ITB, the Huangsh
Incyte Announces the European Commission Approval of Zynyz® (retifanlimab) for the First-Line Treatment of Advanced Squamous Cell Carcinoma of the Anal Canal (SCAC)6.3.2026 22:42:00 CET | Press release
- Zynyz® (retifanlimab) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) is the first systemic treatment for adult patients with advanced SCAC in Europe- The EC approval is based on results of the POD1UM-303 study which showed that adult patients with advanced SCAC achieved significantly improved progression-free survival with Zynyz in combination with carboplatin and paclitaxel as a first-line treatment compared to chemotherapy alone.1 Incyte (Nasdaq:INCY) today announced that the European Commission (EC) has approved Zynyz® (retifanlimab) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) for the first-line treatment of adult patients with metastatic or with inoperable locally recurrent squamous cell carcinoma of the anal canal (SCAC). “The EC approval of Zynyz marks an important step forward for patients with advanced SCAC, a rare cancer for which meaningful treatment advances have not occurred in several decades,” said Bill Meur
Dfns Launches Payouts6.3.2026 21:27:00 CET | Press release
Dfns today announced the launch of Payouts, a new API enabling institutions to convert stablecoins to fiat and route payouts across multiple bank accounts while keeping wallet-level governance and controls in place. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260305327930/en/ Convert stablecoins to fiat and settle payouts to bank accounts in 94 countries, today. Solving the problem of single-rail off-ramps Today, most fintechs and institutions still hard-wire a single payout provider into their stack, or rely on vertically integrated models that bundle routing, pricing, custody, and settlement together. That approach may be convenient early on, but it creates structural problems at scale: weak price discovery because there is no competitive pressure on margins, limited auditability because routing decisions are opaque, and operational fragility because a single provider degradation in any corridor requires architectural i
Klarna Group Plc Clarifies Mechanics of March 9 Lock-Up Expiration6.3.2026 20:23:00 CET | Press release
Klarna Group plc (NYSE: KLAR) today issues the following clarification to ensure investors and market participants have accurate information regarding the mechanics of its lock-up expiration on March 9, 2026, the processes required before pre-IPO shares can be traded on the NYSE, and the prior liquidity opportunities already available to shareholders. This release contains only factual descriptions of the Company's share structure and applicable processes. It does not constitute guidance or a projection of any kind regarding future trading volumes, share price, or the intentions of any shareholder and speaks only as of the date of this press release. 1. 335 million locked-up shares — but two different categories Of the 378 million total ordinary shares outstanding, approximately 335 million are subject to lock-up restrictions expiring March 9, 2026. However, these shares fall into two distinct categories governed by separate sets of regulations. A. 159 million shares (48% of locked-up
Lone Star Funds Announces Agreement to Acquire the Capsules & Health Ingredients Division of Lonza Group AG6.3.2026 18:30:00 CET | Press release
Lone Star Funds (“Lone Star”) today announced that an affiliate of Lone Star Fund XII, L.P. has entered into a definitive agreement to acquire the Capsules & Health Ingredients (“CHI”) division of Lonza Group AG. As part of the transaction, Lonza will retain a 40% equity position in the business. Headquartered in Basel, Switzerland, CHI operates globally across the Americas, Europe and Asia Pacific. The business comprises three segments: Hard Empty Capsules: leading global manufacturer of gelatin and plant-based capsules offering a broad range of innovative solutions for pharmaceutical and nutraceutical customers. Dosage Form Solutions: end-to-end development and manufacturing platform serving nutraceutical and pharmaceutical customers. Health Ingredients: provider of branded, science-backed nutrition ingredients serving joint health, energy and active lifestyle markets. Lone Star believes CHI is a high-quality, globally recognized platform with strong technical capabilities, different
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom