Align Technology Launches Align X-ray Insights, an AI Computer-Aided Detection Software, in the European Union and United Kingdom
The Solution to be Showcased at 2025 IDS Dental Show in Cologne, Germany
Align Technology, Inc. (“Align”) (Nasdaq: ALGN), a leading global medical device company that designs, manufactures, and sells the Invisalign® System of clear aligners, iTero™ intraoral scanners, and exocad™ CAD/CAM software for digital orthodontics and restorative dentistry, today announced the launch in European Union countries and the United Kingdom of Align X-ray Insights, a new software-based (CADe*) computer aided detection solution that uses artificial intelligence (AI) to automatically analyze 2D radiographs.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250310448632/en/
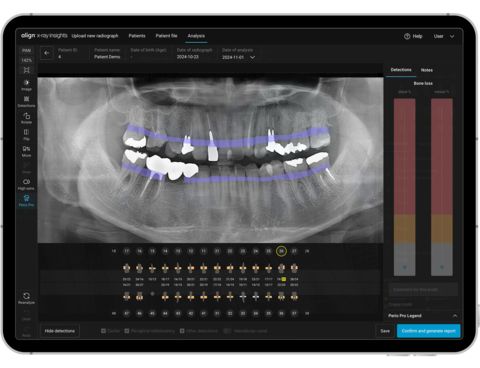
Align X-ray Insights, an AI Computer-Aided Detection Software (Photo: Business Wire)
As part of the Align™ Digital Platform, Align X-ray Insights software is designed to support doctors to diagnose dental and oral health conditions, standardize analysis, streamline workflows, and improve patient engagement. A recent survey among early doctor users of the technology showed it helped 95% of them in communicating patient oral health conditions. Furthermore, 91% agreed that, when shown to patients, it improved patient trust and treatment acceptance of restorative procedures.1
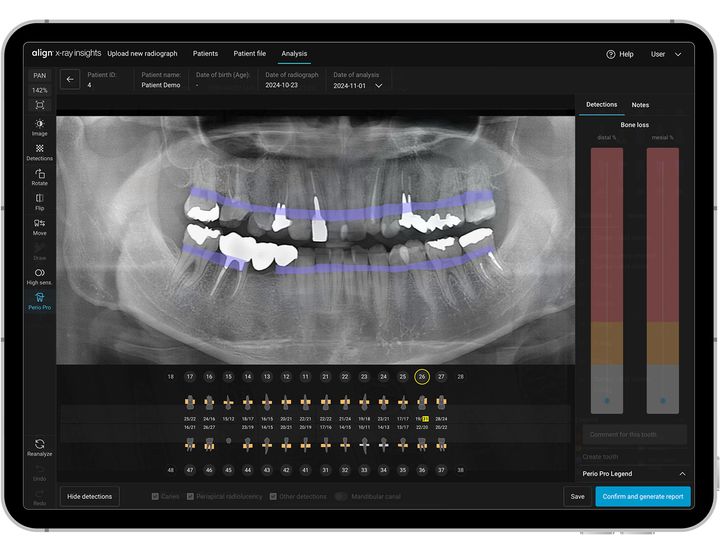
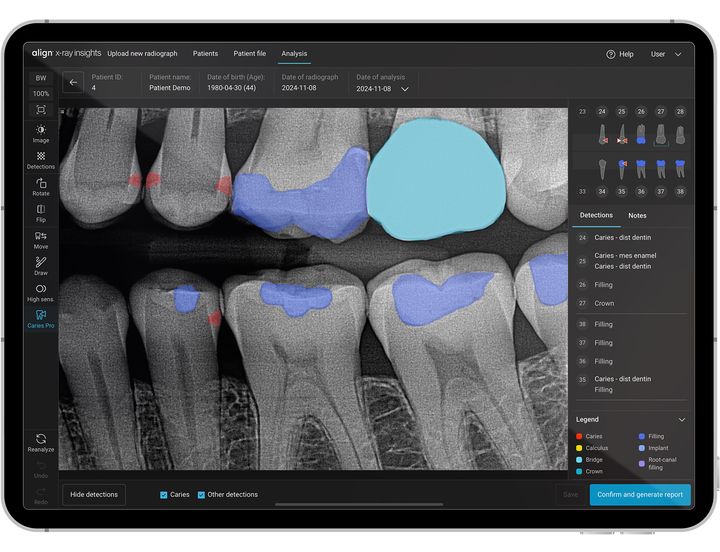
After confirming diagnosis, doctors can use the Align X-ray Insights detailed report, automatic tooth charting, and color overlays of radiographic abnormalities for patient education and treatment planning. The AI detection functionalities include caries, periapical radiolucencies and periodontal bone loss and other conditions.
Align X-ray Insights – alongside Align™ Oral Health Suite, a comprehensive digital suite providing a clinical framework to empower engaging oral health conversations – further reinforces Align’s commitment to dental diagnostics by launching an X-ray diagnostic digital solution that can enhance the already available NIRI (Near Infra-Red Imaging) technology with iTero intraoral scanners and Oral Health Suite’s capabilities, with the objective to support and advance doctor treatment planning decisions and priorities, based on the patient records available.
“Align X-ray Insights represents a significant advancement in our digital restorative dentistry solutions with broader patient applicability,” said Simon Beard, Align Technology executive vice president and managing director, Europe, Middle East, and Africa (EMEA). “By integrating AI into radiographic analysis, we are empowering doctors with more precise diagnostic capabilities to improve their patient outcomes. This launch underscores our commitment to innovation and our ongoing efforts to expand our digital platform.”
“It is fantastic to see Align X-ray Insights being integrated into Align’s digital platform – bringing diagnostic AI capabilities to dentists all over the world. When we started our journey towards AI in dental diagnostics, we had hoped to improve patient outcomes via better diagnostics and treatment decisions,” said Professor Falk Schwendicke, director of the Dental Clinic at University of Munich. “Since then, we learned that further value lies in Align X-ray Insights by fostering patient communication, and facilitating comprehensive, systematic reporting. Combining this with the products and services already available to Align users is a leap forward for digital dentistry.”
Align Technology acquired dentalXrai GmbH in 2022 and has since worked on integrating its flagship product into the Align Digital Platform. Align X-ray Insights, a cloud-based software, can be accessed either from any desktop or tablet or through an integration with iTero. The desktop / tablet version becomes generally available in the European Union and United Kingdom as of March 25, 2025, and will be presented at the IDS tradeshow in Cologne, Germany on March 25-29, 2025. The integration of Align X-ray Insights with iTero™ intraoral scanners is underway and will commence limited market release soon. Align X-ray Insights received regulatory clearance in Europe, the UK, Canada and New Zealand, with further availability in other countries planned, pending approvals such as 510K clearance in the United States of America.
For more information, interested customers can visit www.alignxrayinsights.com. As part of the launch offer, doctors can benefit from a 60-day free-trial.
*CADe = computer-aided detection
- Based on a survey of n=24 doctors (19 GP / 5 orthodontists) using Align™ X-ray Insights in Europe (FR, UK, IT, DE, ES, NL, DK and LV) who were part of a limited market release. Doctors indicated their agreement with different statements using a 4-point scale from strongly agree to strongly disagree. Statements included “Align X-ray Insights, when shown to patients, increased treatment acceptance of restorative procedures.” (91% agreement), “Align X-ray Insights streamlines the dental diagnostic workflow, helping me save valuable time.” (70% agreement), “Align X-ray Insights contributes to higher trust when shown to patients during treatment option discussions.” (91% agreement) and “Align X-ray Insights helps me communicate patient oral health conditions and treatment options.” (95% agreement). Align X-ray Insights is intended to be used in conjunction with an evaluation by the doctor and should not be solely relied upon to make or confirm a diagnosis. Data on file at Align Technology as of January 2025.
About Align Technology, Inc.
Align Technology designs and manufactures the Invisalign® System, iTero™ intraoral scanners and services, and exocad™ CAD/CAM software. These technology building blocks enable enhanced digital orthodontic and restorative workflows to improve patient outcomes and practice efficiencies for over 271.6 thousand doctor customers and is key to accessing Align’s 600 million consumer market opportunity worldwide. Over the past 28 years, Align has helped doctors treat approximately 19.5 million patients with the Invisalign System and is driving the evolution in digital dentistry through the Align™ Digital Platform, our integrated suite of unique, proprietary technologies and services delivered as a seamless, end-to-end solution for patients and consumers, orthodontists and GP dentists, and lab/partners. Visit www.aligntech.com for more information.
For additional information about the Invisalign System or to find an Invisalign doctor in your area, please visit www.invisalign.com. For additional information about the iTero digital scanning system, please visit www.itero.com. For additional information about exocad dental CAD/CAM offerings and a list of exocad reseller partners, please visit www.exocad.com.
Invisalign, iTero, exocad, Align, and Align Digital Platform are trademarks of Align Technology, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250310448632/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Andersen Consulting forbedrer sine organisatoriske udviklingskapaciteter med Omni HR Consulting1.8.2025 19:22:00 CEST | Pressemeddelelse
Andersen Consulting udvider sine kompetencer inden for menneskelige ressourcer gennem en samarbejdsaftale med Omni HR Consulting, et sydafrikansk konsulentfirma med speciale i løsninger til forretnings- og personaleudvikling. Omni HR Consulting tilbyder en komplet pakke af tjenester, der omfatter organisationsudvikling, præstationsrådgivning, akkrediteret uddannelse, kompetenceudvikling og ledelsesprogrammer gennem sit Business and Leadership Academy. Virksomheden samarbejder med kunderne om at designe og implementere løsninger, der retter sig mod medarbejdernes kompetencer, optimering af resultater og strategisk tilpasning og understøttes af en konsekvent tilgang til projektledelse og overholdelse af sydafrikanske kvalitetsstandarder. "Hos Omni tror vi på, at effektiv udvikling starter med forståelse af konteksten," siger administrerende direktør Lize Moldenhauer. "Vi arbejder tæt sammen med vores kunder for at udvikle skræddersyede løsninger, der skaber målbare fremskridt – hvad ente
DevvStream Deploys Crypto Treasury with Initial Bitcoin and Solana Purchases; Intends to Expand Credit Facility to $300M1.8.2025 16:00:00 CEST | Press release
DevvStream Corp. (Nasdaq: DEVS) (“DevvStream” or the “Company”), a leading carbon management firm specializing in the development, investment, and sale of environmental assets, today announced the initial deployment of its crypto treasury strategy with purchases of Bitcoin ($BTC) and Solana ($SOL), funded by a portion of the first (US)$10 million tranche of its (US)$300 million senior secured convertible notes facility with Helena Global Investment Opportunities 1 Ltd. These acquisitions represent the operational launch of DevvStream’s digital treasury strategy, designed to combine institutional-grade liquidity with blockchain infrastructure. The Company believes Bitcoin provides a liquid, non-correlated store of value and that Solana’s high-throughput network supports the Company’s long-term objectives in, and the industry’s move towards, sustainability-linked tokenization. In parallel, DevvStream announced its intention to increase its existing Equity Line of Credit (ELOC) to (US)$30
BEYOND Launches PASSO, a Sculptural Icon on Palm Jumeirah1.8.2025 15:17:00 CEST | Press release
BEYOND Developments, the forward-thinking real estate brand shaping lifestyle destinations by the sea, has unveiled PASSO, a sculptural waterfront development located on the prestigious West Crescent of Palm Jumeirah. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250801880717/en/ PASSO by BEYOND, a Sculptural Icon on Palm Jumeirah. (Photo: AETOSWire) As BEYOND’s first flagship beyond its masterplan in Dubai Maritime City, PASSO marks a defining milestone in the company’s strategic growth to one of the world’s most iconic and desirable destinations. The project launched with a spectacular Palm Jumeirah event featuring Dubai’s first-ever “screens in the sky” show, a 13-minute performance with over 4,000 drones blending immersive visuals and live stage action. Comprising two sculptural towers, Avita and Bella, PASSO offers 625 residences in a refined mix of layouts. From one-bedroom retreats and two-to-four-bedroom-plus lifest
LevelBlue Completes Acquisition of Aon’s Cybersecurity and IP Litigation Consulting Groups1.8.2025 14:00:00 CEST | Press release
Strategic deal enhances LevelBlue's cybersecurity offerings, solidifying its position as the world’s largest leading independent, pure-play MSSP LevelBlue, a global leader in cloud-based, AI-driven managed security services, today announced the completion of its acquisition of Aon’s (NYSE: AON) Cybersecurity and Intellectual Property (IP) Litigation consulting groups, including the renowned cybersecurity firm, Stroz Friedberg, and Elysium Digital. With this completion the consulting group will operate as Stroz Friedberg, a LevelBlue company. This strategic acquisition adds elite cyber and high-tech IP litigation consulting expertise to the LevelBlue portfolio, which includes a globally recognized platform of approximately 300 technology professionals with deep relationships across Fortune 500 companies, 80 percent of the Am Law 100, and most of the UK’s top 20 law firms. As a result, LevelBlue will significantly fortify its incident response and advisory capabilities, while expanding i
SBC Medical to Announce Q2 2025 Financial Results and Hold Conference Call on August 13, 20251.8.2025 14:00:00 CEST | Press release
SBC Medical Group Holdings Incorporated (Nasdaq: SBC) (“SBC Medical” or the “Company”), a global franchise and provider of services for aesthetic clinics, today announced that it will report its Q2 2025 financial results on Wednesday, August 13, 2025, before the U.S. market opens. The Company will hold a conference call on Wednesday, August 13, 2025 at 8:30 am Eastern Time (or Wednesday, August 13, 2025 at 9:30 pm Japan Time) to discuss the financial results and take questions live. Please register in advance of the conference using the link provided below. https://edge.media-server.com/mmc/p/ukc9sp9j It will automatically direct you to the registration page of “SBC Q2 2025 Financial Results Presentation.” Please follow the steps to enter your registration details, then click “Submit.” Upon registration, you will be able to access the dedicated Conference Call viewing site. In addition to viewing the conference call, this site provides access to information about the speakers as well a
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom