PHC and CCRM Collaborate to Develop Primary T-Cell Expansion Culture Processesto Enhance Efficiency and Improve Cell Quality
PHC Corporation has signed a Master Collaboration Agreement with CCRM to work together on the development of primary T-cell(*1) expansion culture processes that will seek to accelerate the manufacturing of cell and gene therapy (CGT) products. This joint initiative will integrate “LiCellGrowTM(*2), PHC’s cell expansion system under development, with CCRM’s deep knowledge of regenerative medicine and biomanufacturing to establish new culture processes to improve cell culture efficiency and quality for CGTs.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250209611769/en/
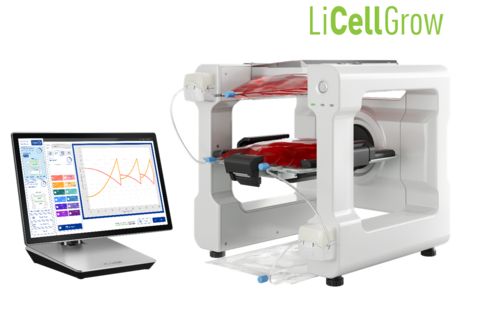
*For research purposes only. Anticipated visual image of LiCellGrow. All features subject to change. (Graphic: Business Wire)
Primary T-cells are used in process development and manufacturing for CGTs, such as in CAR-T cell therapy.(*3) However, primary T-cells derived directly from patients often exhibit significant variability in growth rates and quality, making it challenging for researchers to ensure stable cell counts and maintain quality throughout the culture process. To address these challenges and improve the quality of cell-based therapeutics, better cell culture processes are needed.
Chikara Takauo, Director of PHC and Head of the Biomedical Division that leads the company’s Life Science business, commented: "We are delighted to begin this joint research and development initiative with CCRM, a leader with 14 years of experience in the commercialization of regenerative medicine and CGT. By combining the technologies and expertise of both of our organizations, we aim to advance the manufacturing processes for cell-based therapeutics and cell culture technologies, contributing to the early practical application of CGT."
PHC has developed proprietary In-Line monitoring technology to track key indicators of cell metabolism in real-time, which can help researchers address issues like cell quality and reproducibility, and establish optimal cell culture methods. This technology enables precise, continuous measurement of glucose uptake and lactate production during cell culture, providing a more precise understanding of changes in cell metabolism over time than is possible to observe using traditional sampling methods. In 2024, PHC launched the live-cell metabolic analyzer "LiCellMoTM(*4)" incorporating this technology in the United States, Canada, Europe and some Asian markets including Japan, China, Singapore and Taiwan.
Building on this technology, the company is also developing "LiCellGrow," a cell expansion system designed to exchange media automatically based on the metabolic state of the cells and to maintain the culture environment in an optimal state. PHC aims to further expand its product lineup to seamlessly support research, process development, and commercial manufacturing of cell-based therapeutics.
"We are excited to collaborate with PHC to unlock new possibilities in cell culture,” explained Michael May, President and CEO of CCRM. “Technology development partnerships, like this one, are key to advancing the industry and making CGT more cost effective, and therefore more accessible to patients around the world.”
The joint research with CCRM will allow PHC to analyze culture conditions using "LiCellGrow" to establish optimal culture processes for primary T-cells. The collaboration will seek to accelerate LiCellGrow’s development, contributing to improved cell quality, enhanced manufacturing efficiency, and cost reduction in the production of cell-based therapeutics.
(*1) Primary T cells, or autologous T cells, refer to T cells that are directly isolated from the body. T cells are part of the immune system and develop from stem cells in the bone marrow. They help protect the body from infection and may help fight cancer.
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/t-cell
https://www.cancer.gov/search/results?swKeyword=autologous
(*2) URL: http://www.phchd.com/us/biomedical/licellgrow
(*3) CAR T-cell therapy is a type of treatment in which a patient's T cells (a type of immune system cell) are changed in the laboratory so they will attack cancer cells.
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy
(*4) URL: https://www.phchd.com/us/biomedical/live-cell-metabolic-analyzer
About the Biomedical Division of PHC Corporation
Established in 1969, PHC Corporation is a Japanese subsidiary of PHC Holdings Corporation (TOKYO: 6523), a global healthcare company that develops, manufactures, sells, and services solutions across diabetes management, healthcare solutions, diagnostics and life sciences. The Biomedical Division supports the life sciences industry helping researchers and healthcare providers in around 110 countries and regions through its PHCbi-branded laboratory and equipment and services including CO2 incubators and ultra-low temperature freezers.
www.phchd.com/global/phc
About PHC Holdings Corporation (PHC Group)
PHC Holdings Corporation (TOKYO: 6523) is a global healthcare company with a mission of contributing to the health of society through healthcare solutions that have a positive impact and improve the lives of people. Its subsidiaries (referred to collectively as PHC Group) include PHC Corporation, Ascensia Diabetes Care, Epredia, LSI Medience Corporation, Wemex and Mediford. Together, these companies develop, manufacture, sell and service solutions across diabetes management, healthcare solutions, diagnostics and life sciences. PHC Group’s consolidated net sales in FY2023 were JPY 353.9 billion with global distribution of products and services in more than 125 countries.
www.phchd.com/global
About CCRM and OmniaBio
CCRM is a global, public-private partnership headquartered in Canada. It has received funding from the Government of Canada, the Province of Ontario, and leading academic and industry partners. CCRM supports the development of regenerative medicines and associated enabling technologies, with a specific focus on cell and gene therapy. A network of researchers, leading companies, investors, and entrepreneurs, CCRM accelerates the translation of scientific discovery into new companies and marketable products for patients with specialized teams, dedicated funding, and unique infrastructure. In 2022, CCRM established OmniaBio Inc., a commercial-stage CDMO for manufacturing cell and gene therapies. CCRM is hosted by the University of Toronto. Visit us at ccrm.ca.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250209611769/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
MultiBank Group Confirms $MBG Token TGE Set for July 22, 202512.7.2025 11:14:00 CEST | Press release
MultiBank Group, the world’s largest financial derivatives institution has officially announced that the Token Generation Event (TGE) for its highly anticipated $MBG Token will take place on July 22, 2025. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250712404220/en/ MultiBank Group has officially announced that the Token Generation Event (TGE) for its highly anticipated $MBG Token will take place on July 22, 2025. This milestone will mark the full activation of the $MBG Token on the blockchain, enabling holders to view and manage their balances across supported platforms. Following the token minting, users will be able to trade $MBG via MultiBank.io, the Group’s regulated crypto exchange and Uniswap, the world’s leading decentralized platform. The $MBG Token has garnered global attention for its rare combination of real-world utility, institutional backing, and strong deflationary mechanics. It is underpinned by $29 billi
Elegen and Nutcracker Therapeutics to Pilot First Fully Cell-Free Manufacturing Process for RNA-based Personalized Cancer Therapeutics11.7.2025 14:00:00 CEST | Press release
Fully cell-free process aims to further democratize personalized cancer therapeutic manufacturing with shorter turnaround times and negligible bioburden and endotoxin risks. Elegen, a global leader in next-generation DNA manufacturing, and Nutcracker Therapeutics, a global leader in next-generation RNA design and manufacturing, today announced the launch of a pilot program to demonstrate the industry’s first fully synthetic, cell-free manufacturing platform for RNA-based personalized cancer therapeutics (PCTs). The pilot marks another step toward making PCTs more accessible, timely, and scalable. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250711152688/en/ As late-stage PCT clinical trials progress and therapy developers work to create the next generation of PCTs, the speed, reliability, scaling and cost of traditional production methods pose a major challenge. Specifically, the first step of DNA template production is hi
$MBG Token Pre-Sale Set for July 15 — Only 7 Million Tokens Available at $0.3511.7.2025 10:17:00 CEST | Press release
MultiBank Group, the world’s largest financial derivatives institution headquartered in Dubai, has confirmed that its highly anticipated $MBG Token pre-sale will go live on July 15, with demand expected to be intense. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250711737311/en/ With only 7 million $MBG tokens up for grabs at an exclusive entry price of $0.35, this is a rare opportunity to secure early access to what many are calling the year’s most powerful utility asset. With only 7 million tokens up for grabs at an exclusive entry price of $0.35, this is a rare opportunity to secure early access to what many are calling the year’s most powerful utility asset. Early participants can join simultaneously on MultiBank.io, the Group’s regulated crypto exchange, and Uniswap, the world’s leading decentralized platform. Supported by $29 billion in real assets and powered by over $35 billion in daily turnover, $MBG is engineered
Live Story Raises €2.7 Million to Revolutionize the Digital Experience11.7.2025 10:05:00 CEST | Press release
With a round led by Vertis, the next-generation CMS platform accelerates its focus onAI, performance, and European expansion. Target: surpass €10M in recurring revenueby 2027. Live Story, the tech company founded by Stefano Mocellini, has closed a €2.7 million seed round led by Vertis, one of Italy’s leading early-growth venture capital firms. The funding will support the company’s international expansion and technological development, with a clear goal: to exceed €10 million in annual recurringrevenue by 2027. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250711335560/en/ “We invested in Live Story because it addresses one of the major inefficiencies in digital commerce: the slow and rigid management of visual and narrative content,” says Alessandro Pontari, Partner at Vertis SGR. “The platform helps brands drastically reduce their time-to-market through a visual CMS that integrates seamlessly with any tech stack. In a wor
With a Score of 84 out of 100, Sagemcom Is Awarded the EcoVadis Platinum Medal: a Prestigious Recognition of its CSR Commitment11.7.2025 09:00:00 CEST | Press release
Sagemcom Group is proud to announce that it has been awarded, for the third time, the Platinum Medal by EcoVadis, the highest distinction granted by the leading global platform for assessing Corporate Social Responsibility (CSR) performance. This medal places Sagemcom in the top 1% of companies evaluated worldwide, across all industries. With a score of 84 out of 100, Sagemcom reaffirms its position as a committed leader in ecological transition, business ethics, sustainable supply chain management, and social responsibility. “The EcoVadis Platinum Medal is more than just an award — it is the recognition of our collective efforts to embed sustainable development principles at the heart of our corporate strategy and culture,” says Sylvaine Couleur, Executive Vice President, CSR & Communication. “Achieving this level demonstrates that our commitments are tangible, impactful, and internationally recognized. This distinction strengthens our determination to further advance and expand our C
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom