Regula Launches Full Support for Digital Travel Credentials in Latest Software Update
15.1.2025 08:00:00 CET | Business Wire | Press release
Regula, a global developer of forensic devices and identity verification solutions, has updated its Regula Document Reader SDK. Now, the software fully supports the new Digital Travel Credential (DTC) format, aligned with the International Civil Aviation Organization (ICAO) standards. This enhancement enables governments, airlines, and border control authorities worldwide to process travel documents with unmatched security, efficiency, and ease—whether on-site or remotely.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250115990355/en/
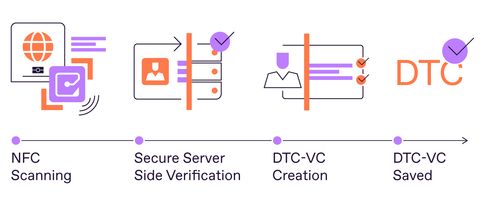
With Regula Document Reader SDK, travelers can create their own digital credentials by extracting a Virtual Component (DTC-VC) from a physical electronic identity document like ePassport. (Graphic: Regula)
The DTC is a secure digital solution that streamlines travel experiences. It consolidates key personal information into a single virtual document that travelers can store on their mobile devices or upload to their digital wallets and share whenever needed. The main goal of the DTC is to facilitate clearance procedures during travel and ensure that people are eligible to enter their destination before they board a flight.
The ICAO has defined three types of DTC, each offering varying levels of convenience and security for travelers.
- DTC Type 1 allows travelers to create their own digital credentials by extracting a Virtual Component (DTC-VC) from a physical electronic identity document, stored on their personal device. However, travelers must still carry the original document for identity verification.
- DTC Type 2, issued by authorities, combines a cryptographically linked Virtual Component (DTC-VC) and a Physical Component (DTC-PC). This format adds an extra layer of security while maintaining a connection to the physical document.
- DTC Type 3 represents the future of travel identification: a fully digital document issued directly by authorities. Unlike the other types, it eliminates the need for a physical ID, streamlining identity verification for a completely digital experience.
Now, with Regula Document Reader SDK, users can create and reprocess DTC-VC from ePassports, and verify it by passing DTC-VC data as input. Also, support for handling DTC-PC has been introduced. The updated Regula Document Reader SDK can:
- read the document’s RFID chip with a smartphone or passport reader and create DTC-VC;
- recognize, read, and verify DTC-VC with a smartphone, passport reader, or server;
- read DTC-PC with a smartphone or passport reader, parse its data, and verify it.
The updated Regula Document Reader SDK is equipped with advanced features that fully support DTC implementation.
- Trustworthy NFC verification. First and foremost, it provides trusted server-side NFC verification of the ID so it ensures accurate and trustworthy DTC-VC creation. Since all the data from the chip can be verified on a secure server, there is no need to question the reliability of the checks performed by a mobile device (which is prone to manipulation). Such an approach ensures that the virtual component of a traveler’s document is secure and taken from an authentic ID.
- Compliance with ICAO guidelines and technical reports. Regula Document Reader SDK not only verifies DTCs but also guarantees that each DTC fully complies with ICAO guidelines and technical reports. This makes Regula’s solution an indispensable tool for airlines and governments so they can be confident in the validity of travelers’ DTCs.
- Future-ready technology. In addition to supporting DTC-VC, Regula’s technology is fully compatible with handling DTC-PC. Looking further ahead, Regula is ready to process DTC Type 3, a digital passport that is expected in the next several years.
To facilitate the fast and smooth global application of DTCs, Regula Document Reader SDK relies on the most comprehensive identity document template database, which is owned and maintained by Regula. Currently, it contains more than 14,000 ID templates from 251 countries and territories, and it’s constantly growing.
Regula Document Reader SDK is designed for easy integration into third-party applications, allowing clients to incorporate this advanced DTC-ready technology seamlessly into their existing systems. With customizable options, businesses can adapt the solution to meet their unique operational requirements while providing end users with a secure, streamlined digital experience.
“We see that the world is rapidly moving to embrace digital IDs. According to the Forrester Consulting study commissioned by Regula earlier in 2024, nearly half of businesses around the world, 42%, are actively integrating digital IDs into their systems. For the Aviation sector, this rate is even higher: 50%. By ensuring full DTC support in the latest update of Regula Document Reader SDK, we are helping our clients to smoothly transition to the future of travel, where secure, digital-first solutions redefine the passenger experience,” says Ihar Kliashchou, Chief Technology Officer at Regula.
To learn more about the capabilities of the updated Regula Document Reader SDK, read the release documentation on Regula’s website.
About Regula
Regula is a global developer of forensic devices and identity verification solutions. With our 30+ years of experience in forensic research and the most comprehensive library of document templates in the world, we create breakthrough technologies for document and biometric verification. Our hardware and software solutions allow over 1,000 organizations and 80 border control authorities globally to provide top-notch client service without compromising safety, security, or speed. Regula has been repeatedly named a Representative Vendor in the Gartner® Market Guide for Identity Verification. Learn more at www.regulaforensics.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250115990355/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
AI Meets Traditional Culture: Huangshan Captures Widespread Attention at ITB Berlin7.3.2026 10:22:00 CET | Press release
Huangshan, one of China’s most iconic scenic destinations, drew significant attention at this year’s ITB by presenting a compelling fusion of traditional Chinese culture and cutting-edge artificial intelligence under the slogan “The world of Huangshan is for the world.” This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260307909978/en/ International visitor admires Huangshan cultural and creative exhibits at the Huangshan stand during ITB Berlin. Located in eastern China’s Anhui Province, Huangshan is famed for its “Five Natural Wonders” — fantastic pines, grotesque rocks, sea of clouds, hot spring and winter snow. The mountain is widely regarded as one of China’s greatest mountain landscapes. It is also a rare natural heritage site that simultaneously holds multiple international designations, including UNESCO World Cultural and Natural Heritage status, a UNESCO Global Geopark and a World Biosphere Reserve. At ITB, the Huangsh
Incyte Announces the European Commission Approval of Zynyz® (retifanlimab) for the First-Line Treatment of Advanced Squamous Cell Carcinoma of the Anal Canal (SCAC)6.3.2026 22:42:00 CET | Press release
- Zynyz® (retifanlimab) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) is the first systemic treatment for adult patients with advanced SCAC in Europe- The EC approval is based on results of the POD1UM-303 study which showed that adult patients with advanced SCAC achieved significantly improved progression-free survival with Zynyz in combination with carboplatin and paclitaxel as a first-line treatment compared to chemotherapy alone.1 Incyte (Nasdaq:INCY) today announced that the European Commission (EC) has approved Zynyz® (retifanlimab) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) for the first-line treatment of adult patients with metastatic or with inoperable locally recurrent squamous cell carcinoma of the anal canal (SCAC). “The EC approval of Zynyz marks an important step forward for patients with advanced SCAC, a rare cancer for which meaningful treatment advances have not occurred in several decades,” said Bill Meur
Dfns Launches Payouts6.3.2026 21:27:00 CET | Press release
Dfns today announced the launch of Payouts, a new API enabling institutions to convert stablecoins to fiat and route payouts across multiple bank accounts while keeping wallet-level governance and controls in place. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260305327930/en/ Convert stablecoins to fiat and settle payouts to bank accounts in 94 countries, today. Solving the problem of single-rail off-ramps Today, most fintechs and institutions still hard-wire a single payout provider into their stack, or rely on vertically integrated models that bundle routing, pricing, custody, and settlement together. That approach may be convenient early on, but it creates structural problems at scale: weak price discovery because there is no competitive pressure on margins, limited auditability because routing decisions are opaque, and operational fragility because a single provider degradation in any corridor requires architectural i
Klarna Group Plc Clarifies Mechanics of March 9 Lock-Up Expiration6.3.2026 20:23:00 CET | Press release
Klarna Group plc (NYSE: KLAR) today issues the following clarification to ensure investors and market participants have accurate information regarding the mechanics of its lock-up expiration on March 9, 2026, the processes required before pre-IPO shares can be traded on the NYSE, and the prior liquidity opportunities already available to shareholders. This release contains only factual descriptions of the Company's share structure and applicable processes. It does not constitute guidance or a projection of any kind regarding future trading volumes, share price, or the intentions of any shareholder and speaks only as of the date of this press release. 1. 335 million locked-up shares — but two different categories Of the 378 million total ordinary shares outstanding, approximately 335 million are subject to lock-up restrictions expiring March 9, 2026. However, these shares fall into two distinct categories governed by separate sets of regulations. A. 159 million shares (48% of locked-up
Lone Star Funds Announces Agreement to Acquire the Capsules & Health Ingredients Division of Lonza Group AG6.3.2026 18:30:00 CET | Press release
Lone Star Funds (“Lone Star”) today announced that an affiliate of Lone Star Fund XII, L.P. has entered into a definitive agreement to acquire the Capsules & Health Ingredients (“CHI”) division of Lonza Group AG. As part of the transaction, Lonza will retain a 40% equity position in the business. Headquartered in Basel, Switzerland, CHI operates globally across the Americas, Europe and Asia Pacific. The business comprises three segments: Hard Empty Capsules: leading global manufacturer of gelatin and plant-based capsules offering a broad range of innovative solutions for pharmaceutical and nutraceutical customers. Dosage Form Solutions: end-to-end development and manufacturing platform serving nutraceutical and pharmaceutical customers. Health Ingredients: provider of branded, science-backed nutrition ingredients serving joint health, energy and active lifestyle markets. Lone Star believes CHI is a high-quality, globally recognized platform with strong technical capabilities, different
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom