SamanTree Medical Highlights Research Showing 67% Reduction in Reoperation Rates for Breast-Conserving Surgery at the 2024 SABCS
11.12.2024 15:00:00 CET | Business Wire | Press release
SHIELD study shows reoperation rates dropped from 30% to 10% with use of the Histolog® Scanner, highlighting its potential to improve oncologic surgery and patient outcomes
SamanTree Medical, a leader in oncologic surgical imaging innovation, today announced promising results from the SHIELD study, which will be presented by researchers on Dec. 12 at the 2024 San Antonio Breast Cancer Symposium (SABCS). The findings show that the Histolog® Scanner, a confocal laser microscope, reduced reoperation rates during breast-conserving surgery (BCS) from 30% to 10%, representing a 67% reduction. The innovative imaging device also demonstrated significant accuracy in detecting positive margins with high sensitivity and specificity.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241211957581/en/
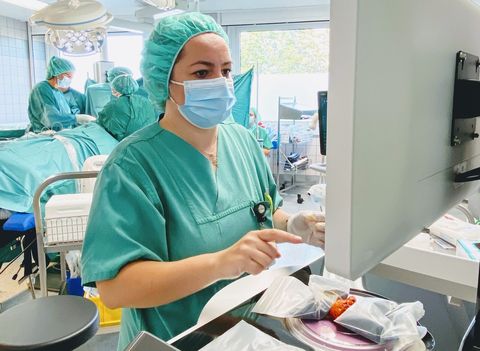
Dr. Mariana-Felicia Sandor, first author of the SHIELD study: Reoperation Rate in Breast-Conserving Surgery using Confocal Histolog® Scanner for Intraoperative Tissue Assessment, performs real-time, high-resolution tissue assessments during breast-conserving surgery (BCS) using the FDA-cleared Histolog Scanner. SHIELD study data indicates the innovative imaging device reduced reoperation rates during BCS from 30% to 10%, representing a 67% reduction. (Photo: Business Wire)
“The results of the SHIELD study impressively demonstrate a proof-of-concept of the Histolog Scanner in patients with breast cancer and also confirm the non-interventional POLARHIS study,” said Professor Michael Patrick Lux, M.D., principal investigator of the SHIELD study and Head of the Department of Gynecology and Obstetrics at St. Vincenz Hospital, Paderborn, Germany. “The data are absolutely relevant for clinical care, as the system can reduce the re-section rate in a clinically relevant and significant way, thus optimizing oncological safety, cosmetics, and the economic aspects of care.”
The prospective study, conducted at St. Vincenz Hospital, evaluated the impact of the Histolog Scanner on reducing reoperation rates during BCS. Fifty patients with invasive breast cancer and/or ductal carcinoma in situ (DCIS) were enrolled, and reoperation rates were compared to historical data from the POLARHIS study, a retrospective observational study performed at the same breast center by the same surgical team using standard intraoperative margin assessment techniques.
The results showed a dramatic reduction in reoperation rates from 30% in the POLARHIS study to 10% in the SHIELD study, representing a 67% improvement when the Histolog Scanner was used. Moreover, the Histolog Scanner outperformed standard techniques by achieving a sensitivity of 80.9% and a specificity of 99.5% for positive margin detection, compared to the combined sensitivity of 17.4% and specificity of 97.3% achieved with standard methods, as seen in the SHIELD study.
"These outstanding results underscore the potential of the Histolog Scanner to enhance oncologic surgical outcomes significantly," said Olivier Delporte, CEO of SamanTree Medical. "By enabling real-time, high-resolution tissue microstructure assessments during BCS, our technology addresses a critical unmet need, reducing reoperation rates and improving patient outcomes.
"With the recent FDA clearance, we are pleased to make this groundbreaking technology available to surgeons throughout the U.S. The Histolog Scanner sets a new standard for precision and care in oncologic surgery, marking a significant milestone in our mission to transform oncologic surgery and improve patient care globally," continued Delporte.
About SamanTree Medical
Headquartered in Liège, Belgium, with U.S. regional headquarters in Boston, SamanTree Medical SA is dedicated to improving oncologic surgery through innovative imaging solutions. Its flagship product, the Swiss-developed Histolog Scanner, features massive parallel confocal microscopy, a cutting-edge technology that expands the imaging field up to 40,000 times larger than standard confocal microscopy. The breakthrough device enables surgeons and pathologists to visualize large, fresh tissue samples in real-time with exceptional resolution and accuracy, making it uniquely suited for intraoperative environments in oncologic surgery. Founded in 2010 based on innovations from the École Polytechnique Fédérale de Lausanne (EPFL), a leading Swiss research institute renowned for engineering and technological advancements, SamanTree Medical is committed to improving surgical precision and outcomes by enabling real-time fresh tissue imaging. More information may be found here.
Click here for Important Safety Information.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241211957581/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Estithmar Holding Pays the Third Semi-Annual coupon of the 8.75% Sukuk Tranche12.3.2026 21:50:00 CET | Press release
Estithmar Holding Q.P.S.C. has paid the third semi-annual coupon of its Qatari Riyal-denominated Sukuk (first tranche), at an annual profit rate of 8.75%. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260312880092/en/ Estithmar Holding Pays the Third Semi-Annual coupon of the 8.75% Sukuk Tranche (Photo: AETOSWire) The first tranche, part of the company’s broader Sukuk program valued at QAR 3.4 billion and listed on the London Stock Exchange’s International Securities Market, was issued in August 2024. The issuance attracted a diverse pool of institutional investors including banks, insurance companies, and asset managers, with strong interest from both government-affiliated and private institutions. This demand reflects growing investor confidence in Estithmar Holding’s ability to deliver sustained value to stakeholders. EstithmarHolding was recently included in the FTSE Russell Global Equity Index, in Qatar’s Mid-Cap segme
Andersen Consulting styrker sine kompetencer i samarbejde med Acumen Learning12.3.2026 21:36:00 CET | Pressemeddelelse
Andersen Consulting udvider sin platform gennem en samarbejdsaftale med Acumen Learning, en amerikansk virksomhed, der specialiserer sig i træning i forretnings- og økonomiforståelse med henblik på lederudvikling og salgsresultater. Acumen Learning blev stiftet i 2002 og samarbejder med Fortune 500-virksomheder for en bedre finansiel forståelse, strategisk tænkning og beslutningstagning på alle niveauer. Med udgangspunkt i principperne fra deres bestsellerbøger "Seeing the Big Picture" og "Business Acumen for Sales Success" klæder deres programmer ledere og teams på til at afstemme beslutninger med virksomhedsstrategier, fremme resultater og styrke kunderelationer. Acumen Learning er målrettet brancher som sundhedssektoren, energi og teknologi og giver fagfolk mulighed for at omsætte forretningsviden til håndgribelige resultater. "Hos Acumen Learning er vores mission at styrke det enkelte menneske ved at skabe forretningskyndige fagfolk, der gør en forskel i deres karrierer," udtalte K
REPLY: The Board of Directors Approved the Draft Financial Statements for the Year 202512.3.2026 15:38:00 CET | Press release
All economic indicators are positive.Consolidated turnover of €2,483.6 million (€2,300.5 million in 2024);EBITDA at €467.6 million (€410.6 million in 2024);EBIT at €391.7 million (€330.4 million in 2024)Group net profit at €250.9 million (€211.1 million in 2024)Approval of the proposed dividend distribution of €1.35 per share. Today the Board of Directors of Reply S.p.A. [MTA, STAR: REY] approved the draft financial statement for the year 2025, which will be submitted for approval to the Shareholders’ Meeting to be held in first call in Turin on 23 April 2026. The Reply Group closed 2025 with a consolidated turnover of €2,483.6 million, an increase of 8.0% compared to €2,300.5 million in 2024. All indicators are positive for the period. Consolidated EBITDA was €467.6 million, an increase of 13.9% compared to €410.6 million at December 2024. EBIT, from January to December, was at €391.7 million, which is an increase of 18.5% compared to €330.4 million at December 2024. The Group net pro
LZE GmbH Introduces Fraunhofer’s RFicient® Technology to the Market12.3.2026 14:51:00 CET | Press release
LZE GmbH is expanding its technology transfer portfolio and making the RFicient® ultra-low-power wake-up receiver technology from the Fraunhofer Institute for Integrated Circuits IIS available for the first time as a standard chip for close-to-production industrial applications. The solution enables energy-efficient IoT designs that remain continuously reachable while consuming only microamps – a key step for long-lasting, low-maintenance IoT products. LZE GmbH drives technology transfer to market: standard chip availability for close-to-production applications As a bridge between research and industry, LZE GmbH is making it easier for companies to access innovative technologies and helping them to quickly and reliably transform new developments into market-ready solutions. With RFicient®-IC (FH101RF), LZE is providing another high-tech product that comes directly from Fraunhofer research and can now be ordered in volume and integrated into close-to-production product development for t
Owkin Creates New Spin out Waiv, Formerly Owkin Dx, With $33M Financing12.3.2026 14:30:00 CET | Press release
Investment lead by OTB Ventures and Alpha Intelligence CapitalWaiv develops AI-powered precision testing to better identify and stratify patients in the clinic and in clinical trials, transforming patient careWaiv extends Owkin’s strategy of real-world validation for its AI Owkin, the AI company on a mission to solve the complexity of biology, today announced the spin out of Waiv, formerly known as Owkin Dx. The move follows significant investor interest and positions Waiv to bring AI-powered precision testing for better identification of patients in the clinic and in clinical trials, to transform patient care. This follows on from the successful launch of Bioptimus, an Owkin incubated company, in February 2024. Waiv translates AI innovation into real-world clinical impact, developing tests that predict biomarkers and patient outcomes, including RlapsRisk BC for prognostic risk profiling. With multiple tests already in use in clinical settings, its deployment platform Destra, and colla
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom