SamanTree Medical Highlights Research Showing 67% Reduction in Reoperation Rates for Breast-Conserving Surgery at the 2024 SABCS
11.12.2024 15:00:00 CET | Business Wire | Press release
SHIELD study shows reoperation rates dropped from 30% to 10% with use of the Histolog® Scanner, highlighting its potential to improve oncologic surgery and patient outcomes
SamanTree Medical, a leader in oncologic surgical imaging innovation, today announced promising results from the SHIELD study, which will be presented by researchers on Dec. 12 at the 2024 San Antonio Breast Cancer Symposium (SABCS). The findings show that the Histolog® Scanner, a confocal laser microscope, reduced reoperation rates during breast-conserving surgery (BCS) from 30% to 10%, representing a 67% reduction. The innovative imaging device also demonstrated significant accuracy in detecting positive margins with high sensitivity and specificity.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241211957581/en/
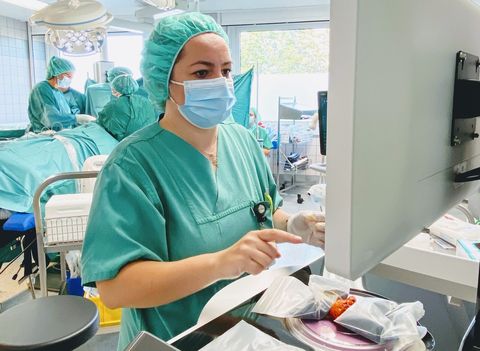
Dr. Mariana-Felicia Sandor, first author of the SHIELD study: Reoperation Rate in Breast-Conserving Surgery using Confocal Histolog® Scanner for Intraoperative Tissue Assessment, performs real-time, high-resolution tissue assessments during breast-conserving surgery (BCS) using the FDA-cleared Histolog Scanner. SHIELD study data indicates the innovative imaging device reduced reoperation rates during BCS from 30% to 10%, representing a 67% reduction. (Photo: Business Wire)
“The results of the SHIELD study impressively demonstrate a proof-of-concept of the Histolog Scanner in patients with breast cancer and also confirm the non-interventional POLARHIS study,” said Professor Michael Patrick Lux, M.D., principal investigator of the SHIELD study and Head of the Department of Gynecology and Obstetrics at St. Vincenz Hospital, Paderborn, Germany. “The data are absolutely relevant for clinical care, as the system can reduce the re-section rate in a clinically relevant and significant way, thus optimizing oncological safety, cosmetics, and the economic aspects of care.”
The prospective study, conducted at St. Vincenz Hospital, evaluated the impact of the Histolog Scanner on reducing reoperation rates during BCS. Fifty patients with invasive breast cancer and/or ductal carcinoma in situ (DCIS) were enrolled, and reoperation rates were compared to historical data from the POLARHIS study, a retrospective observational study performed at the same breast center by the same surgical team using standard intraoperative margin assessment techniques.
The results showed a dramatic reduction in reoperation rates from 30% in the POLARHIS study to 10% in the SHIELD study, representing a 67% improvement when the Histolog Scanner was used. Moreover, the Histolog Scanner outperformed standard techniques by achieving a sensitivity of 80.9% and a specificity of 99.5% for positive margin detection, compared to the combined sensitivity of 17.4% and specificity of 97.3% achieved with standard methods, as seen in the SHIELD study.
"These outstanding results underscore the potential of the Histolog Scanner to enhance oncologic surgical outcomes significantly," said Olivier Delporte, CEO of SamanTree Medical. "By enabling real-time, high-resolution tissue microstructure assessments during BCS, our technology addresses a critical unmet need, reducing reoperation rates and improving patient outcomes.
"With the recent FDA clearance, we are pleased to make this groundbreaking technology available to surgeons throughout the U.S. The Histolog Scanner sets a new standard for precision and care in oncologic surgery, marking a significant milestone in our mission to transform oncologic surgery and improve patient care globally," continued Delporte.
About SamanTree Medical
Headquartered in Liège, Belgium, with U.S. regional headquarters in Boston, SamanTree Medical SA is dedicated to improving oncologic surgery through innovative imaging solutions. Its flagship product, the Swiss-developed Histolog Scanner, features massive parallel confocal microscopy, a cutting-edge technology that expands the imaging field up to 40,000 times larger than standard confocal microscopy. The breakthrough device enables surgeons and pathologists to visualize large, fresh tissue samples in real-time with exceptional resolution and accuracy, making it uniquely suited for intraoperative environments in oncologic surgery. Founded in 2010 based on innovations from the École Polytechnique Fédérale de Lausanne (EPFL), a leading Swiss research institute renowned for engineering and technological advancements, SamanTree Medical is committed to improving surgical precision and outcomes by enabling real-time fresh tissue imaging. More information may be found here.
Click here for Important Safety Information.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241211957581/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
DCO Concludes 5th General Assembly with Adoption of the Kuwait Declaration on Responsible AI for Global Digital Prosperity7.2.2026 16:41:00 CET | Press release
The Digital Cooperation Organization (DCO) has concluded its fifth General Assembly, with Member States adopting the Kuwait Declaration on Responsible AI for Global Digital Prosperity and agreeing on actions to advance inclusive, trusted, and scalable digital transformation in the AI age. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260207972901/en/ DCO concludes 5th General Assembly with adoption of the Kuwait Declaration on Responsible AI for Global Digital Prosperity (Photo: AETOSWire) Convened on 4–5 February 2026 under the Presidency of the State of Kuwait, the General Assembly brought together Ministers and Representatives of Member States, alongside Observers, partners, and guest countries, to review progress against the DCO 4-Year Agenda (2025–2028), take joint decisions on multilateral initiatives, and translate shared ambition on AI into delivery. Ministers and representatives reaffirmed their commitment to inclu
Rapid Medical™’s DISTALS Trial Overwhelmingly Positive, Demonstrating Superior Reperfusion with TIGERTRIEVER™ 13 in Medium Vessel Stroke6.2.2026 20:30:00 CET | Press release
TIGERTRIEVER™ 13 is the first device shown to meet safety and effectiveness endpoints for restoring blood flow in smaller but critical areas of the brain, accounting for almost 50% of all ischemic strokes Rapid Medical™, a leading developer of active endovascular devices, today announced late-breaking results from the DISTALS multicenter, randomized controlled trial showing that TIGERTRIEVER™ 13 achieved superior brain tissue reperfusion with an excellent safety profile when compared with medical management in medium vessel occlusion (MVO) stroke. The findings were presented in the main closing session at the 2026 International Stroke Conference (ISC). Top-line results showed that the TIGERTRIEVER™ 13 arm demonstrated 3x more successful reperfusion without symptomatic intracranial hemorrhage (sICH) compared to medical management–86.3% vs 27.7% (p < 0.001). Notably, zero sICH events were reported in the randomized treatment arm treated with TIGERTRIEVER 13. By comparison, sICH rates rep
Al Barari Breaks Ground on The Cape, The Final Chapter of a Legacy6.2.2026 15:46:00 CET | Press release
Al Barari, Dubai’s pioneering nature-led community, has officially marked the groundbreaking of The Cape, its final signature residential development, celebrating two decades of visionary craftsmanship and a continued commitment to creating harmonious living environments rooted in nature. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260206475199/en/ Hazza Zaal, CEO of Al Barari Real Estate Group, alongside the Sales and Construction teams at The Cape Groundbreaking Ceremony, marking a milestone in Al Barari’s final signature development. (Photo: AETOSWire) The milestone ceremony signals the beginning of a landmark chapter for Al Barari, as The Cape represents the culmination of a 20-year legacy defined by intentional design, wellbeing-focused living, and immersive natural landscapes. Located within Dubai’s green heart, The Cape introduces an enriched lifestyle experience surrounded by lush botanical settings, gentle water
Andersen Consulting indgår samarbejdsaftale med Alfa Group6.2.2026 15:41:00 CET | Pressemeddelelse
Andersen Consulting styrker sit udbud inden for cybersikkerhed gennem samarbejdet med Alfa Group, der er en førende teknologivirksomhed med næsten tre årtiers erfaring i at hjælpe organisationer med at beskytte og optimere deres drift. Alfa Group blev grundlagt i 1996 og har hovedsæde i Rom. Virksomheden leverer avancerede løsninger inden for cybersikkerhed, registrering og forebyggelse af svindel, hændelseshåndtering og sårbarhedsstyring samt udvalgte ydelser inden for processtyring. Deres proprietære teknologi, N.O.V.A., er en fuldt integreret administreret tjeneste, der kombinerer Alfa Groups innovationer med tredjepartssystemer for at beskytte digitale infrastrukturer, reducere risici og øge den operationelle effektivitet. Virksomheden betjener kunder på tværs af brancher, herunder finans og forsikring, forsvar og rumfart, telekommunikation, fremstilling, energi og medicinalindustrien. "Vores samarbejde med Andersen Consulting giver os mulighed for at udvide vores rækkevidde og lev
Quantfury and Sandwich Launch Commercial Series to Encourage Better Choices in Retail Trading6.2.2026 15:00:00 CET | Press release
Quantfury Trading Americas Limited (“Quantfury”), a global brokerage offering commission-free trading at real-time spot prices from major exchanges, has partnered with Sandwich, a leading creative agency for tech and product videos, to produce a Social Responsibility Commercial Series. The series of commercials incorporates clear, engaging storytelling to prompt viewers to think about common retail trading behaviors—especially high-energy marketing, gamification of speculation, and heavy focus on potential gains without equal attention to risks—and to choose more thoughtful, informed approaches. Lev Mazur, Founder of Quantfury, said: “It’s a pleasure to work with Sandwich, whose visual storytelling is outstanding. Since day one, Quantfury has aimed to question and improve the standard practices in the global retail trading industry.” Adam Lisagor, Founder of Sandwich, added: “A good investment is a story. Quantfury stood out by wanting to tell a real, interesting story first—rather tha
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom