Orthogon Therapeutics Closes Oversubscribed $5.2M Financing to Advance First Oral Therapy for BK Polyomavirus
Orthogon’s small molecule therapeutics redefine treatment possibilities for BK virus infections.
Orthogon Therapeutics, a developer of novel antiviral medicines, today announced the closing of an oversubscribed financing round, exceeding its $5M target and bringing total funding to over $25M. This funding will accelerate the development of its first-in-class treatments for managing BK virus reactivation in transplant patients.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241210996847/en/
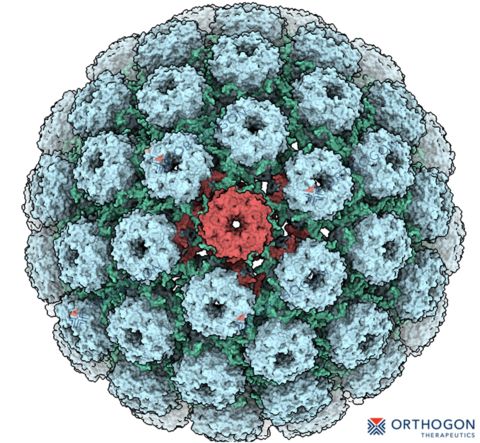
Ultrastructural representation of the BK polyomavirus capsid, featuring 72 pentamers that form its distinct icosahedral symmetry. (Graphic: Business Wire)
The company has successfully developed small molecule drugs that directly target viral proteins essential to the polyomavirus life cycle. Polyomaviruses lack conventional antiviral protein targets and have eluded drug development for decades. Backed by a diversified investor base and a pipeline of drugs with unique mechanisms of action, Orthogon is addressing this area of urgent unmet medical need.
“Our high-affinity small molecule antivirals rival the potency of biologics without their drawbacks,” said Dr. Stephen Weeks, VP of Structural Biology at Orthogon Therapeutics. “This achievement highlights our platform’s ability to target previously undruggable viral proteins.”
This breakthrough coincides with Orthogon’s creation of a pioneering animal model to study BK polyomavirus pathology. Historically, researchers have lacked in vivo insights into BK virus disease progression. This model deepens understanding of disease mechanisms and provides a robust platform for testing therapies, solidifying Orthogon’s leadership in addressing barriers to polyomavirus treatments.
The company’s therapeutic strategy offers the first comprehensive solution to managing the full BK virus risk ladder—from early reactivation, indicated by virus shedding in the urine, to systemic spread and severe complications such as BKVAN, graft dysfunction, and transplant failure. By intervening at the earliest stages of reactivation, this approach extends treatment benefits to more patients, reduces the need for aggressive late-stage interventions, and improves long-term transplant success.
“It is incredibly rewarding to see our drug designs progress from the bench into translational models,” said Ali H. Munawar, Ph.D., CEO of Orthogon Therapeutics. “Our small molecule antivirals offer unique advantages over emerging therapies, overcoming challenges in delivery, stability, and—most importantly—reaching the intracellular sites of viral replication in the kidney. These capabilities, combined with the dosing flexibility of oral administration, enable us to close significant gaps in transplant patient care.”
This strategic raise underscores Orthogon’s disciplined approach to capitalization, enabling focused progress on high-impact milestones and reinforcing investor confidence in its mission to deliver the first orally administered therapy for BK polyomavirus infections.
About BK and Polyomaviruses:
BK virus (BKV), a member of the polyomavirus family, establishes a lifelong, persistent yet silent infection in over 80% of healthy adults. BK virus reactivation occurs in the kidneys of nearly half of all solid organ and stem cell transplant patients, leading to severe complications and graft loss. Other human polyomaviruses, such as JC virus and Merkel Cell polyomavirus, cause fatal progressive multifocal leukoencephalopathy (PML) and aggressive Merkel cell carcinoma.
About Orthogon Therapeutics:
Orthogon leverages advanced structure-based drug design and biophysics to create groundbreaking therapies for challenging drug targets. To learn more, visit www.orthogontherapeutics.com.
Orthogon Therapeutics, LLC is an independent, privately held R&D company affiliated with the Pledge Therapeutics discovery platform. The company is headquartered in the biotech hub of Greater Boston with a branch in Leuven, Belgium. More info at www.pledge-tx.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241210996847/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
TEVIMBRA Approved in U.S. for First-line Treatment of Gastric and Gastroesophageal Junction Cancers in Combination with Chemotherapy27.12.2024 12:00:00 CET | Press release
New indication based on results from a global Phase 3 trial demonstrating TEVIMBRA plus chemotherapy significantly improved overall survival for patients with advanced gastric cancersSecond FDA approval for TEVIMBRA in 2024 BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology company that intends to change its name to BeOne Medicines Ltd., today announced the U.S. Food and Drug Administration (FDA) has approved TEVIMBRA® (tislelizumab-jsgr), in combination with platinum and fluoropyrimidine-based chemotherapy, for the first-line treatment of unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma (G/GEJ) in adults whose tumors express PD-L1 (≥1). “Today’s FDA approval of TEVIMBRA for the treatment of gastric or gastroesophageal junction cancers in PD-L1 positive adult patients marks a significant step forward in our mission to deliver transformative therapies to patients with cancer,” said Mark Lanasa, M.D., Ph.D., Chief Medical
Takeda Announces Approval of HYQVIA® 10% S.C. (Subcutaneous) Injection Set in Japan for Patients with Agammaglobulinemia or Hypogammaglobulinemia27.12.2024 07:00:00 CET | Press release
HYQVIA [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase] is the First and Only Facilitated Subcutaneous Immunoglobulin (fSCIG) Approved in Japan for Agammaglobulinemia and HypogammaglobulinemiaAdministration of Recombinant Human Hyaluronidase Prior toImmunoglobulin Facilitates Subcutaneous Infusion of Larger Volumes, Potentially Reducing Frequency and Giving Patients More FlexibilityApproval Expands Takeda’s Portfolio of Differentiated Immunoglobulin Therapies and Reflects the Company’s Commitment to Bring High-Quality Plasma-Derived Therapies to Patients in Japan Takeda (TSE:4502/NYSE:TAK) today announced that the Japanese Ministry of Health, Labour and Welfare has approved the use of HYQVIA® [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase] in patients with agammaglobulinemia or hypogammaglobulinemia1, disorders characterized by very low or absent levels of antibodies and an increased risk of serious recurring infection caused by pr
Kolmar Korea Named One of the TIME’s World’s Best Companies for Sustainable Growth26.12.2024 15:00:00 CET | Press release
Ranks 125th Among 500 Companies Around the GlobeTop 10% in the Environmental Sector Among 3,000 Major Global Companies Kolmar Korea (KRX: 024720) has been recognized by TIME Magazine as one of the World’s Best Companies for Sustainable Growth 2025. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241226863828/en/ Kolmar Korea on TIME's World's Best Companies in Sustainable Growth 2025 (Image: Kolmar Korea) On November 27, TIME, in collaboration with a global market research firm Statista, unveiled a list of the top 500 companies worldwide, demonstrating exceptional sustainable growth. Kolmar Korea took place 125th around the globe and 6th among Korean companies. Notably, it was the only Korean cosmetics company on the list, which featured a total of 23 Korean companies. The rankings were based on an evaluation of revenue growth, financial stability, and environmental impact, each contributing equally to a final score out of 10
Seoul Semiconductor: Philips Lighting Products Ordered to Recall 7-Year-Old Items26.12.2024 08:00:00 CET | Press release
The German District Court of Düsseldorf, on November 19 ruled in favor of Seoul Semiconductor (KOSDAQ:046890) in the patent infringement lawsuits, and also ordered that products manufactured by Philips Lighting and sold since March 2017 be recalled and destroyed. The Court also ruled that a fine of up to €250,000 would be imposed for each violation of this order. On December 17, the German Federal Patent Court also affirmed the validity of these patents, which solidifies the strength of many related patents. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241225151382/en/ Application Examples with CRI 70 or Higher (Photo: Seoul Semiconductor) These court orders relate to the core technology used to achieve CRI 70 (Color Rendering Index 70%) or higher, applicable to all home lighting, automotive lighting products, IT flash, and backlights. Since the effects of these judgments are applicable to all products infringing on the pa
ispace-EUROPE and the Italian Space Agency (ASI) Sign Payload Services Agreement to Transport a Laser Retroreflector Array (LaRA2) on the Moon Surface26.12.2024 08:00:00 CET | Press release
Agreement Marks Significant Step Towards Increasing Italy’s Contribution to advancing Lunar Exploration ispace EUROPE S.A. (ispace-EUROPE), the Luxembourg-based subsidiary of ispace, inc., and the Italian Space Agency (ASI) have signed a payload services agreement to transport a Laser Retroreflector Array (LaRA2) to enable accurate position measurements on the Moon via laser ranging experiments, the two organizations announced today. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241225137548/en/ The shape of LaRA2, a palm-sized dome (Photo: Business Wire) The agreement marks the first full-scale contract between ispace-EUROPE and ASI, with both organizations looking to joint future lunar development. LaRA2 is a small, robust, and lightweight instrument built to work without any power source and to survive the harsh surface conditions on the Moon for an extended period of time. It features a precise array of retroreflectors
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom