IQM Quantum Computers Unveils Development Roadmap Focused on Fault-tolerant Quantum Computing by 2030
IQM's development roadmap aims to achieve fault-tolerance by 2030 and outlines a path to scaling up to 1 million qubits with combined quantum error reduction and error correction. The roadmap is underpinned by merging IQM's two processor topologies Star and Crystal for hardware efficient error correction as well as an open modular software stack for HPC integration. IQM also demonstrates early industry use cases in quantum machine learning, simulation of quantum systems and optimization.
IQM Quantum Computers (IQM), a global leader in superconducting quantum computing, today announced its development roadmap with technical milestones targeting fault tolerant quantum computing by 2030, while enabling a dedicated Noisy Intermediate-Scale Quantum (NISQ) approach for near-term usage.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241113963297/en/
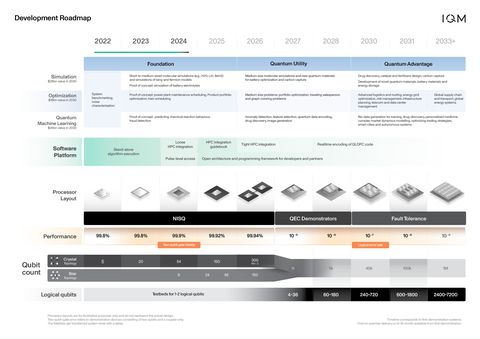
IQM Quantum Computers Development Roadmap (Graphic: Business Wire)
Since its start, IQM has successfully delivered full-stack quantum computers based on its first three processor generations. IQM's 12-year roadmap reflects its vision for pioneering quantum solutions through novel algorithmic approaches, modular software integration, and scalable hardware advancements. It leverages the company's ability to design and fabricate next-generation quantum processors with seamless integration into full-stack systems controlled by an open software stack.
IQM's unique co-design capabilities steer the roadmap towards efficient error-correction implementations with high system performance by merging IQM's two processor topologies IQM Star and IQM Crystal. To enable the roadmap, IQM systematically invests in its R&D, testing and fabrication facilities to boost technology scaling up to 1 million qubits while maintaining high qubit quality and gate fidelity.
To support the developer community and to ease the usage of quantum computing, IQM will also enable tight high-performance computing (HPC) integration and create a special software development kit (SDK). Open interfaces will empower the ecosystem, including quantum error mitigation, co-develop libraries and use-cases on IQM’s quantum computers.
The company aims to achieve quantum advantage across multiple industry domains, focusing on quantum simulations, optimization, and quantum machine learning. According to a McKinsey report, these selected use-cases will unlock a value potential of more than US$28 billion by 2035.
Quantum advantage will be provided by fully error-corrected systems with hundreds to thousands of high-precision logical qubits, for which error correction will be enabled by efficiently implementing novel quantum low-density parity-check (QLDPC) codes. This approach reduces the hardware overhead by a factor of up to 10 compared to surface code implementation.
Furthermore, IQM is targeting high-precision logical qubits with error rates below 10^-7, enabling quantum advantage for applications demanding exceptional accuracy, such as in chemistry and materials science.
“We are implementing Quantum low-density parity-check (QLDPC) codes through a novel chip topology, enabled by our uniquely connected Star topology, long-distance couplers and a very compact approach for advanced packaging and signal routing,” said Dr. Jan Goetz, Co-Founder and Co-CEO of IQM Quantum Computers. “This underlines our commitment to hardware efficiency, enabling a feasible and scalable pathway to fault tolerance combined with an open and modular software architecture.”
Goetz emphasizes that the company’s proprietary cleanroom facilities will support the fabrication of complex processors with unique long-range connections, facilitating high-performance quantum processors.
To this end, IQM will implement novel solutions for advanced packaging and 3D integration to ensure scalability while maintaining its ambitious goals to reduce error rates, while its large-scale processors will be built up in a modular way and powered by cryogenic electronics. The results are reduced heat load, strongly miniaturized packaging solutions, and reduced cost per qubit. These features will result in more performant and affordable products for IQM's customers in the HPC and enterprise market.
Offering on-premises and cloud access, IQM has been specializing in integrating quantum systems into HPC centers since 2020. The latest is Germany’s first hybrid quantum computer at the Leibniz Supercomputing Centre.
IQM aims to explain further details of the roadmap in future publications, blog posts, and at industry and academic events.
About IQM Quantum Computers:
IQM is a global leader in designing, building, and selling superconducting quantum computers. IQM provides both on-premises full-stack quantum computers and a cloud platform to access its computers anywhere in the world. IQM customers include the leading high-performance computing centres, research labs, universities and enterprises which have full access to IQM's software and hardware. IQM has over 280 employees with offices in Espoo, Madrid, Munich, Paris, Palo Alto, Singapore and Warsaw.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241113963297/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
SES Completes Acquisition of Intelsat, Creating Global Multi-Orbit Connectivity Powerhouse17.7.2025 07:30:00 CEST | Press release
New leading multi-orbit space company with a network of 120 GEO+MEO satellites and access to LEO constellations enables SES to better serve its customers SES, a leading space solutions company, today announced the completion of its highly value accretive acquisition of Intelsat, creating a strengthened global satellite operator with an expanded fleet of 120 satellites across two orbits. The newly combined company will leverage its skilled teams with deep vertical expertise to deliver integrated multi-orbit, multi-band satellite and connectivity solutions to businesses and governments around the world, creating a stronger multi-orbit operator with ~60% of revenue in high-growth segments. With a world-class network including approximately 90 geostationary (GEO), nearly 30 medium earth orbit (MEO) satellites, strategic access to low earth orbit (LEO) satellites, and an extensive ground network, SES can now deliver connectivity solutions utilising complementary spectrum bands including C-,
Galderma Unveils Final Nine-Month Data Showing Lasting Efficacy and Patient Satisfaction With Its Injectable Aesthetics Portfolio When Addressing Facial Aesthetic Changes After Medication-Driven Weight Loss17.7.2025 07:00:00 CEST | Press release
Final data from a first-of-its-kind clinical trial confirm that the combination of Sculptra® and Restylane® Lyft™ or Contour™* delivers sustained improvement in facial aesthetic appearance for patients experiencing facial volume loss due to medication-driven weight loss1 Following interim analysis at three months presented earlier this year, a six-month extension study was conducted to capture the durability of treatment effects after nine months1 Galderma continues to lead the response to addressing the aesthetic concerns of patients experiencing medication-driven weight loss, leveraging its deep dermatological expertise and working closely with healthcare professionals to meet evolving patient needs Galderma has revealed positive final data from a phase IV first-of-its-kind trial exploring the benefits of Restylane Lyft or Contour in combination with Sculptrato address the aesthetic concerns of patients experiencing facial volume loss associated with medication-driven weight loss.1 T
Global New Material International: Driving a Green Future Through Innovation17.7.2025 04:41:00 CEST | Press release
As global economic integration accelerates, materials science—the foundation of modern industry—is undergoing a profound transformation. Global New Material International Holdings Limited (GNMI) stands at the forefront of this evolution. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250716430383/en/ Colored Building-Integrated Photovoltaic modules developed by CQV, a subsidiary of GNMI. At the recent 2nd Sino-European Corporate ESG Best Practice Conference, GNMI was honored with the “Best Practice in Technological Innovation” award for its outstanding commitment to innovation and green development. The judging panel praised the company’s achievements, noting that “GNMI’s innovations have gained broad international recognition. With a clear strategy focused on green manufacturing and sustainability, the company offers viable solutions to reduce dependence on non-renewable resources while advancing eco-friendly production.” T
Andersen Consulting styrker sin platform med tilføjelsen af Baufest16.7.2025 20:07:00 CEST | Pressemeddelelse
Andersen Consulting udvider sine kompetencer inden for digital transformation gennem en samarbejdsaftale med Baufest, et globalt firma inden for digitalt produktdesign og -udvikling. Med mere end 30 års erfaring og kontorer i hele Amerika og Europa er Baufest anerkendt for at kombinere banebrydende teknologi med en dybdegående forståelse af forretningsstrategi og brugeroplevelse. Firmaet tilbyder et omfattende udvalg af tjenester, der omfatter forretningsdesign, udvikling af digitale produkter, data og anvendt AI og cybersikkerhed. Baufest betjener virksomhedskunder på tværs af nøgleindustrier som bank og finans, detailhandel, energi og sundhedspleje med fokus på at skabe målbare forretningsresultater gennem smidig, skalerbar og etisk teknologipraksis. "Vores samarbejde med Andersen Consulting er et vigtigt skridt fremad for de tjenester, vi tilbyder," siger Ángel Pérez Puletti, administrerende direktør for Baufest. "Andersens fulde pakke af specialiserede tjenester bygger på Baufests
ProAmpac Releases 2025 Sustainability Impact Report: A Decade of Flexibility. A Future of Possibilities16.7.2025 18:54:00 CEST | Press release
ProAmpac, a global leader in flexible packaging and material science, announces the release of its 2025 Sustainability Impact Report. This year’s theme, A Decade of Flexibility. A Future of Possibilities. celebrates the company’s decade-long journey of sustainable innovation and sets the foundation for its forward-looking climate and packaging goals. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250716418035/en/ ProAmpac's 2025 Sustainability Impact Report “As we celebrate ten years of growth and innovation, we remain focused on what lies ahead: accelerating circular packaging, lowering our carbon footprint, and creating shared value for our employees, customers, communities, and partners. Our journey is far from over, but with every step, our purpose becomes clearer, and our momentum stronger,” said Greg Tucker, founder, vice-chairman, and chief executive officer of ProAmpac. Key highlights from the 2025 Impact Report incl
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom