AMIVAS Launches Europe’s First and Only Licensed Severe Malaria Treatment
Artesunate AMIVAS is now available for purchase across Europe and U.K. through Nordic Prime of Denmark. Artesunate AMIVAS, approved by EU Commission and U.K.’s Medicine and Healthcare Product Regulatory Agency to treat severe malaria both in children and adults in Europe, is the only fully licensed such product. Annually, about 1,250 individuals in Europe are diagnosed with severe malaria; most cases are military personnel deployed to malaria-endemic regions or civilian travellers returning from a visit to those regions. Severe malaria has a mortality rate approaching 100 per cent when left untreated; infant, children, pregnant women are among those at highest risk
AMIVAS Ireland Ltd (AMIVAS), a biopharmaceutical company focused on developing, commercialising, manufacturing and distributing artesunate for the treatment of severe malaria, today announced the launch of Artesunate AMIVAS in Europe and the U.K.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241017001569/en/
(Photo: Business Wire)
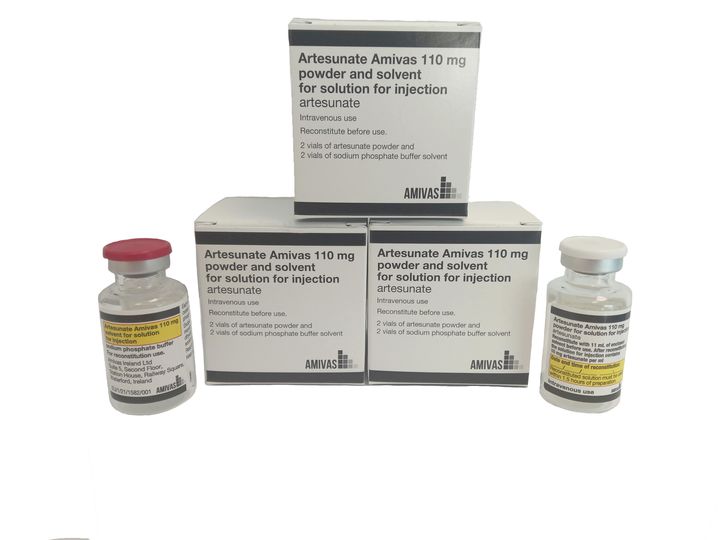
Artesunate AMIVAS is the first and only product licensed, manufactured in a regulated setting, and approved to treat severe malaria in the EU, European Area (EEA), and the U.K. Severe malaria is a disease that impacts an estimated 1,250 European travelers each year. Artesunate AMIVAS is sold as a 110-milligram powder and solvent combined to create an injectable solution.
Artesunate AMIVAS is now available from the Scandinavian distributor, Nordic Prime in Denmark, Finland, Sweden and Norway. To order, see https://www.nordicprime.dk/h
“The launch of Artesunate AMIVAS across Europe means that, for the first time, travelers to and from malaria-endemic regions of the world, and military personnel deployed there, now have access to a licensed, approved severe malaria treatment that could save their lives if needed, said Laura Walsh, AMIVAS Operations Director. “Because of the well-established safety and efficacy profile of Artesunate AMIVAS, healthcare practitioners can now be confident when treating an adult or child diagnosed with severe malaria.”
“It is gratifying to be launching Artesunate AMIVAS in Europe after its successful launch in the United States,” said Sean Power, AMIVAS Director, Ireland. “The AMIVAS mission is focused on bringing safe, effective, fully licensed and approved treatments to patients in need in order to extend and improve their lives. This launch milestone is clear proof of our commitment to that pledge.”
The U.S. Food and Drug Administration approved Artesunate for Injection™ in May, 2020 to treat severe malaria in adults and in children. AMIVAS (US) holds the license.
The European Union Commission awarded approval to AMIVAS Ireland to commercialise Artesunate Amivas in the EU and EEA in late 2021. Approval was granted by the U.K. MHRA in April, 2022.
About Severe Malaria
Malaria is a febrile disease caused by Plasmodium parasites and is usually transmitted by mosquitos. In 2020, almost half of the global population was at risk of contracting it. Over 400,000 deaths are recorded each year from the disease, with sub-Saharan Africa’s population being most at risk. In Europe, 50 years after eradication, malaria is still a major health concern. While most infections in Europe are related to international travel, climatic changes are foreseen to enhance the risk for locally transmitted malaria infections in Europe. In Europe, peaks in the number of malaria cases occur July to September. Since the vast majority of malaria cases are imported, this could partly be linked to travellers returning from summer holidays. Malaria can progress to severe malaria, at which point the mortality rate approaches 100 percent if left untreated. Infants and children under five, pregnant women, and people with low immunity are at highest risk of developing severe malaria. Artesunate has been shown to improve survival rates, with particular benefit for patients with high parasitaemia. Complications of severe malaria include severe anaemia and signs of end-organ damage, such as coma (cerebral malaria), lung complications, hypoglycaemia (low glucose blood levels), or acute kidney injury.
Travelers can protect themselves before, during and after travel (https://www.ecdc.europa.eu/en/malaria).
About Artesunate AMIVAS
Intravenous artesunate has been the global standard of care for severe malaria for more than 20 years. Artesunate AMIVAS is approved for the initial treatment of severe malaria in adults and children by the U.S. Food and Drug Administration, the European Medicines Association, and the Medicine and Healthcare Product Regulatory Agency.
Artesunate is associated with up to a 34.7 percent reduction in risk of mortality compared with quinine. Supplied as a sterile powder, Artesunate can be safely stored at room temperature. Artesunate is easily prepared for use in one step.
Artesunate AMIVAS is superior to standard intravenous quinine in the most important clinical parameter, mortality. Artemisinins, the active ingredient in Artesunate AMIVAS, are the fastest-acting clinical anti-malarial compounds. They can be administered intravenously only when formulated to do so.
About AMIVAS
AMIVAS, based in Nassau, Delaware, in the United States, is a post-approval biopharmaceutical company, founded with a mission to improve and extend human lives by discovering, developing, and distributing new best-in-class medicines. AMIVAS responded to the urgent need for a United States- and European-based firm to assume responsibility for regulated manufacture and distribution of Artesunate after quinidine gluconate was discontinued in the U.S. in 2019. The Company achieved U.S. Food and Drug Administration approval for Artesunate for Injection – its first commercial product – in May, 2020. Artesunate for Injection is indicated for the treatment of severe malaria in adults and children. AMIVAS is committed to being the global leader in the battle against infectious diseases, driving scientific discovery and breakthroughs that will redefine the possibilities of critical medicines.
For more information, visit AMIVAS.com and follow AMIVAS on LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241017001569/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Nordic Group B.V. Through Its Subsidiary Nordic Pharma, Inc. (U.S.), Continues Its Introduction of LACRIFILL® Canalicular Gel, a Novel Therapy for Dry Eye, During American Academy of Ophthalmology (AAO) 202417.10.2024 15:00:00 CEST | Press release
Nordic Pharma to share this innovation and its pivotal study article in the Journal of Cataract and Refractive Surgery (JCRS) with ophthalmology leaders Nordic Pharma, Inc., a subsidiary of Nordic Group B.V., launched LACRIFILL Canalicular Gel, a novel therapy for dry eye in the U.S. in April and will continue its introduction to leading ophthalmologists during AAO 2024. LACRIFILL Canalicular Gel is a cross-linked hyaluronic acid derivative and is FDA-cleared to temporarily block tear drainage by the occlusion of the canalicular system. LACRIFILL Canalicular Gel allows patient’s eyes to be bathed by their own natural tears. It is customized for each individual patient and provides a full fill of the canalicular system. LACRIFILL Canalicular Gel is administered through a simple in-office procedure, which is reimbursed through an existing CPT code (68761). The results last for six months. “We are delighted to be representing Nordic Pharma at AAO,” said Phil Gioia, President of the U.S. t
DriveWealth Bolsters Connectivity for Institutional Broker-Dealers, Enhancing Global Reach and Operational Efficiency17.10.2024 15:00:00 CEST | Press release
Expanded EMS integrations and telecom support will reduce integration times for BDs and enhance trading capabilities and market access DriveWealth, a leading financial technology platform providing Brokerage-as-a-Service, today announced its end-to-end integration with multiple execution management system (EMS) platforms, including Bloomberg EMSX, LSEG Autex and TRAFiX. With these integrations, institutional broker-dealers can connect with DriveWealth’s platform and benefit from streamlined trade booking, reduced integration times, and seamless access to advanced features. “Our goal is to make it turnkey for any institutional broker-dealer to connect to DriveWealth’s existing technology stack without modifying existing systems or infrastructure,” said Aaron Sokasian, Chief Technology Officer at DriveWealth. “With improved connectivity, brokerage firms can experience superior execution quality and extended trading capabilities at a competitive price, all while utilizing the platforms th
Westlake Global Compounds Sites Receive Silver Medal from EcoVadis17.10.2024 15:00:00 CEST | Press release
Westlake Corporation (NYSE: WLK) today announced that Westlake Global Compounds businesses and operating sites outside of the U.S. - Westlake Compounds Holdings, S.A.S. - have been awarded the esteemed EcoVadis Silver Medal, reflecting the company’s commitment to improved environmental sustainability and corporate social responsibility. This accolade places these Westlake Global Compounds sites among the top 15% of companies assessed by EcoVadis globally over the past year. EcoVadis is the world’s largest and most trusted provider of business sustainability ratings and evaluates companies globally on their corporate social responsibility performance using international standards like the Global Reporting Initiative, the United Nations Global Compact, and ISO 26000. “Receiving the EcoVadis Silver Medal is a significant milestone for Westlake Global Compounds and underscores our commitment to environmental stewardship and ethical business practices,” said Bridget Confait-Smith, associate
Samsung Develops Industry’s First 24Gb GDDR7 DRAM for Next-Generation AI Computing17.10.2024 14:41:00 CEST | Press release
New GDDR7 offers industry-leading capacity and speed of over 40Gbps, significantly raising the bar for graphics DRAM powering future applicationsValidation with major GPU customers to begin this year, production slated for early next year Samsung Electronics Co., Ltd., the world leader in advanced memory technology, today announced it has developed the industry’s first 24-gigabit (Gb) GDDR71 DRAM. In addition to the industry’s highest capacity, the GDDR7 features the fastest speed, positioning itself as the optimum solution for next-generation applications. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241017969736/en/ Samsung Develops Industry’s First 24Gb GDDR7 DRAM for Next-Generation AI Computing (Graphic: Business Wire) With its high capacity and powerful performance, the 24Gb GDDR7 will be widely utilized in various fields that require high-performance memory solutions, such as data centers and AI workstations, extend
D & P Communications expands multigigabit services with Adtran’s fiber access solution17.10.2024 14:00:00 CEST | Press release
News summary: Regional service provider needed to grow network capacity without overhauling its existing infrastructure Adtran’s open, intelligent fiber platform offers rapid scale, helping D & P Communications expand multigigabit connectivity across Michigan Versatile fiber broadband solution supports Combo PON technology for seamless network upgrades Adtrantoday announced that D & P Communications is harnessing its open and intelligent fiber access technology to bring multigigabit broadband to more subscribers in southern Michigan. The deployment includes Adtran’s scalable fiber broadband solution with Combo PON technology, enabling D & P Communications to efficiently upgrade to symmetrical 10G PON services by seamlessly supporting the install base of GPON subscribers while blanketing the entire fiber-to-the-home (FTTH) network with the higher speed and capacity of symmetric 10Gbit/s XGS-PON. This empowers the service provider to scale quickly to meet rising demand for high-speed bro
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom