AMIVAS Launches Europe’s First and Only Licensed Severe Malaria Treatment
Artesunate AMIVAS is now available for purchase across Europe and U.K. through Nordic Prime of Denmark. Artesunate AMIVAS, approved by EU Commission and U.K.’s Medicine and Healthcare Product Regulatory Agency to treat severe malaria both in children and adults in Europe, is the only fully licensed such product. Annually, about 1,250 individuals in Europe are diagnosed with severe malaria; most cases are military personnel deployed to malaria-endemic regions or civilian travellers returning from a visit to those regions. Severe malaria has a mortality rate approaching 100 per cent when left untreated; infant, children, pregnant women are among those at highest risk
AMIVAS Ireland Ltd (AMIVAS), a biopharmaceutical company focused on developing, commercialising, manufacturing and distributing artesunate for the treatment of severe malaria, today announced the launch of Artesunate AMIVAS in Europe and the U.K.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241017001569/en/
(Photo: Business Wire)
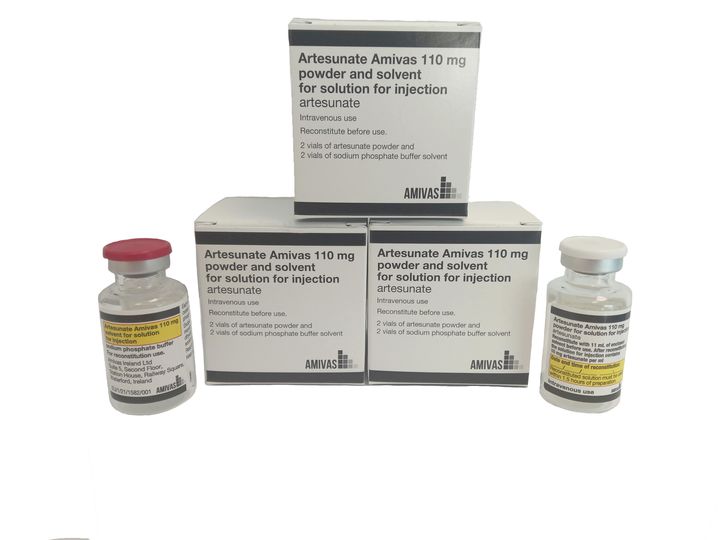
Artesunate AMIVAS is the first and only product licensed, manufactured in a regulated setting, and approved to treat severe malaria in the EU, European Area (EEA), and the U.K. Severe malaria is a disease that impacts an estimated 1,250 European travelers each year. Artesunate AMIVAS is sold as a 110-milligram powder and solvent combined to create an injectable solution.
Artesunate AMIVAS is now available from the Scandinavian distributor, Nordic Prime in Denmark, Finland, Sweden and Norway. To order, see https://www.nordicprime.dk/h
“The launch of Artesunate AMIVAS across Europe means that, for the first time, travelers to and from malaria-endemic regions of the world, and military personnel deployed there, now have access to a licensed, approved severe malaria treatment that could save their lives if needed, said Laura Walsh, AMIVAS Operations Director. “Because of the well-established safety and efficacy profile of Artesunate AMIVAS, healthcare practitioners can now be confident when treating an adult or child diagnosed with severe malaria.”
“It is gratifying to be launching Artesunate AMIVAS in Europe after its successful launch in the United States,” said Sean Power, AMIVAS Director, Ireland. “The AMIVAS mission is focused on bringing safe, effective, fully licensed and approved treatments to patients in need in order to extend and improve their lives. This launch milestone is clear proof of our commitment to that pledge.”
The U.S. Food and Drug Administration approved Artesunate for Injection™ in May, 2020 to treat severe malaria in adults and in children. AMIVAS (US) holds the license.
The European Union Commission awarded approval to AMIVAS Ireland to commercialise Artesunate Amivas in the EU and EEA in late 2021. Approval was granted by the U.K. MHRA in April, 2022.
About Severe Malaria
Malaria is a febrile disease caused by Plasmodium parasites and is usually transmitted by mosquitos. In 2020, almost half of the global population was at risk of contracting it. Over 400,000 deaths are recorded each year from the disease, with sub-Saharan Africa’s population being most at risk. In Europe, 50 years after eradication, malaria is still a major health concern. While most infections in Europe are related to international travel, climatic changes are foreseen to enhance the risk for locally transmitted malaria infections in Europe. In Europe, peaks in the number of malaria cases occur July to September. Since the vast majority of malaria cases are imported, this could partly be linked to travellers returning from summer holidays. Malaria can progress to severe malaria, at which point the mortality rate approaches 100 percent if left untreated. Infants and children under five, pregnant women, and people with low immunity are at highest risk of developing severe malaria. Artesunate has been shown to improve survival rates, with particular benefit for patients with high parasitaemia. Complications of severe malaria include severe anaemia and signs of end-organ damage, such as coma (cerebral malaria), lung complications, hypoglycaemia (low glucose blood levels), or acute kidney injury.
Travelers can protect themselves before, during and after travel (https://www.ecdc.europa.eu/en/malaria).
About Artesunate AMIVAS
Intravenous artesunate has been the global standard of care for severe malaria for more than 20 years. Artesunate AMIVAS is approved for the initial treatment of severe malaria in adults and children by the U.S. Food and Drug Administration, the European Medicines Association, and the Medicine and Healthcare Product Regulatory Agency.
Artesunate is associated with up to a 34.7 percent reduction in risk of mortality compared with quinine. Supplied as a sterile powder, Artesunate can be safely stored at room temperature. Artesunate is easily prepared for use in one step.
Artesunate AMIVAS is superior to standard intravenous quinine in the most important clinical parameter, mortality. Artemisinins, the active ingredient in Artesunate AMIVAS, are the fastest-acting clinical anti-malarial compounds. They can be administered intravenously only when formulated to do so.
About AMIVAS
AMIVAS, based in Nassau, Delaware, in the United States, is a post-approval biopharmaceutical company, founded with a mission to improve and extend human lives by discovering, developing, and distributing new best-in-class medicines. AMIVAS responded to the urgent need for a United States- and European-based firm to assume responsibility for regulated manufacture and distribution of Artesunate after quinidine gluconate was discontinued in the U.S. in 2019. The Company achieved U.S. Food and Drug Administration approval for Artesunate for Injection – its first commercial product – in May, 2020. Artesunate for Injection is indicated for the treatment of severe malaria in adults and children. AMIVAS is committed to being the global leader in the battle against infectious diseases, driving scientific discovery and breakthroughs that will redefine the possibilities of critical medicines.
For more information, visit AMIVAS.com and follow AMIVAS on LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241017001569/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
Nakiki SE: Nakiki SE Evaluates Corporate Bond Offering for Strategic Bitcoin Investment27.8.2025 18:51:00 CEST | Press release
Nakiki SE is currently evaluating the issuance of a corporate bond with a target volume in the mid–single-digit million-euro range. As part of a pre-market sounding process, the Management Board is assessing interest from professional investors. The proceeds of the issuance are intended primarily for the acquisition of Bitcoin. Language: English Company: Nakiki SE Hanauer Landstr. 204 60314 Frankfurt on the Main Germany E-mail: info@nakikifinance.com Internet: https://nakikifinance.com/ ISIN: DE000WNDL300, DE000WNDL318 WKN: WNDL30, WNDL31 Listed: Regulated Market in Frankfurt (General Standard); Regulated Unofficial Market in Berlin, Dusseldorf, Hamburg, Hanover, Munich, Stuttgart, Tradegate Exchange View source version on businesswire.com: https://www.businesswire.com/news/home/20250827593566/en/
Boomi Named a Leader and Secures Top Score in Strategy Category in Latest iPaaS Report by Independent Research Firm27.8.2025 17:30:00 CEST | Press release
AI-driven automation leader receives the highest scores possible in ten criteria Boomi™, the leader in AI-driven automation, today announced it has been named a Leader in The Forrester Wave™: Integration Platform As A Service, Q3 2025. The report evaluated the 10 most significant iPaaS vendors, and Boomi achieved the highest score in thestrategy category among all evaluated providers. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250826324359/en/ Boomi Named a Leader and Secures Top Score in Strategy Category in Latest iPaaS Report by Independent Research Firm The report states that Boomi has reoriented with an “intense focus on AI and APIs,” “receives strong feedback from its partners,” and maintains “clear investment priorities.” Reference customers were impressed by the company’s level of investment in the product and its vision for the future, and look forward to seeing Boomi deliver on its AI roadmap. “We’re honored to
Altasciences and Evidence Matters Announce Strategic Collaboration to Advance AI-Enhanced Text Engineering for Regulatory Writing in Life Sciences27.8.2025 16:00:00 CEST | Press release
Altasciences, a fully integrated CRO/CDMO offering comprehensive early-phase drug development solutions, is pleased to announce a strategic collaboration with Evidence Matters, a pioneer in clinical trial data science and document engineering. This partnership combines Altasciences' real-world drug development expertise with Evidence Matters' innovative, patent-pending Text Engineering technology—a breakthrough that delivers near-deterministic accuracy in regulatory writing by reducing variability and improving the quality, consistency, and speed of documentation. Evidence Matters’ RegulatoryFlow platform (“RegFlow”) and specialized services unify clinical data and documents, simplify workflows, and accelerate the work of key life sciences professionals, from medical writers to regulatory specialists. “We are excited to work alongside Evidence Matters to co-develop technology that directly enhances the quality and efficiency of regulatory writing,” said Nicole Maciolek, Vice President,
Veridas Named a Visionary in the 2025 Gartner® Magic Quadrant™ for Identity Verification27.8.2025 14:34:00 CEST | Press release
Recognised in Gartner research as a leading global IDV vendor, with a fully proprietary stack, certified accessibility, and a vision for reusable, user-centric digital identity. Veridas, a global identity company, has been recognized as a Visionary in the 2025 Gartner® Magic Quadrant™ for Identity Verification, reinforcing its position among the top global IDV vendors. Founded in 2017 and operating globally, Veridas enters the report as one of the fastest-growing companies in the market. Gartner defines Visionaries as vendors that understand where the market is going or have a vision for changing market rules. This positioning validates Veridas’ long-term strategy and highlights its leadership in innovation, ethical design, and user-centric digital identity. At the core of Veridas’ approach is its 100% proprietary technology stack, covering facial biometrics, voice authentication, document verification, age validation, physical access control, and its ID Wallet. By developing all core
Boyd Launches Rack Emulator to Validate Liquid Cooling System Performance for AI Infrastructure27.8.2025 14:05:00 CEST | Press release
Boyd Enables Faster Testing and Validation of Direct-to-Chip Liquid Cooling Loops, Coolant Distribution Units, and Facility Cooling Systems with Boyd’s Rack Emulator Boyd, whose chip-to-ambient liquid cooling technologies make it easier for data center owners and operators to implement new AI infrastructure, announced it launched a new thermal testing tool to help end clients more safely and efficiently deploy liquid cooled data centers and improve time to market. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250827491684/en/ Boyd's new Rack Emulator validates liquid cooling system performance in AI data centers, helping clients more safely and efficiently deploy liquid cooled data centers with improved speed of deployment. Boyd's Rack Emulator simulates the pressure drop and heat dissipation of a rack and uses automation to test coolant distribution units (CDUs) and facility cooling systems to validate thermal performance
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom