KAGA FEI Develops Ultra-small Bluetooth Low Energy Module Compatible with Bluetooth 6.0
10.10.2024 16:00:00 CEST | Business Wire | Press release
KAGA FEI Co., Ltd., a global provider of leading short distance wireless modules, announced today the ES4L15BA1 Bluetooth Low Energy module.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241010420482/en/
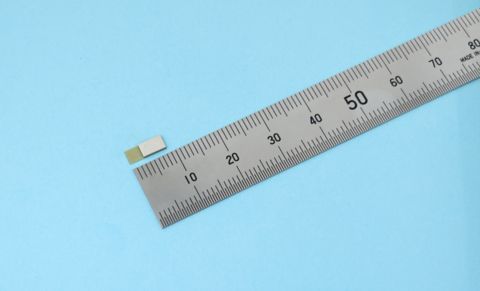
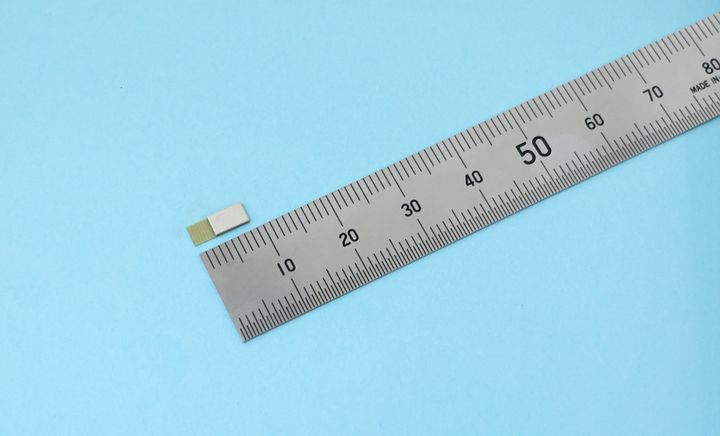
ES4L15BA1 (Photo: Business Wire)
The module has a built-in antenna and has obtained various certifications. Consequently, it reduces the development time and certification costs for next-generation wireless IoT products such as IoT devices, small medical/healthcare products, and wearable devices that require a compact form factor, enabling faster time-to-market.
The ES4L15BA1 also supports channel sounding for accurate distance measurement, providing further convenience for applications requiring reliable distance information or enhanced security based on device distance information, such as unlocking devices based on their proximity.
Furthermore, it supports PSA*1 certification, making it easier to develop IoT devices that meet advanced security requirements. Mass production is scheduled to begin in September 2025. KAGA FEI will continue to respond to market needs and expand its product lineup.
Product Features
-
World’s Smallest Compact Form Factor
The ES4L15BA1 utilizes our proprietary technology to integrate into a 3.25x8.55x1.00mm form factor, achieving the world’s smallest size as an antenna-integrated module compatible with Bluetooth 6.0. -
High Processing Power and Large Memory
The ES4L15BA1 includes an Arm Cortex-M33 processor, RISC-V coprocessor, 1.5MB of non-volatile memory, and 256KB RAM, providing excellent processing performance and high processing efficiency. -
Built-in Antenna and Pre-Certified
Features a built-in antenna, eliminating the need for antenna design. It has obtained Bluetooth qualification and certifications for Radio Law MIC (Japan), FCC (USA), and ISED (Canada), reducing time and costs. In addition, it supports Bluetooth SIG qualification for the latest Bluetooth 6.0 standard, which enables channel sounding function for accurate distance measurement.
Product Availability
Sample | : February 2025 |
Start of mass production | : September 2025 |
About Trademarks
The product names and other proper nouns mentioned herein are trademarks or registered trademarks of their respective companies.
*1. PSA (Platform Security Architecture): PSA is a certification scheme led by Arm. Level 3 is the highest level of certification for protecting IoT devices from physical and software attacks.
KAGA FEI website
https://www.kagafei.com/jp/eng/
Disclaimer
Product specifications, service content, etc. stated in the news release are as of the date of announcement and is subject to change without notice.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241010420482/en/
Subscribe to releases from Business Wire
Subscribe to all the latest releases from Business Wire by registering your e-mail address below. You can unsubscribe at any time.
Latest releases from Business Wire
AI Meets Traditional Culture: Huangshan Captures Widespread Attention at ITB Berlin7.3.2026 10:22:00 CET | Press release
Huangshan, one of China’s most iconic scenic destinations, drew significant attention at this year’s ITB by presenting a compelling fusion of traditional Chinese culture and cutting-edge artificial intelligence under the slogan “The world of Huangshan is for the world.” This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260307909978/en/ International visitor admires Huangshan cultural and creative exhibits at the Huangshan stand during ITB Berlin. Located in eastern China’s Anhui Province, Huangshan is famed for its “Five Natural Wonders” — fantastic pines, grotesque rocks, sea of clouds, hot spring and winter snow. The mountain is widely regarded as one of China’s greatest mountain landscapes. It is also a rare natural heritage site that simultaneously holds multiple international designations, including UNESCO World Cultural and Natural Heritage status, a UNESCO Global Geopark and a World Biosphere Reserve. At ITB, the Huangsh
Incyte Announces the European Commission Approval of Zynyz® (retifanlimab) for the First-Line Treatment of Advanced Squamous Cell Carcinoma of the Anal Canal (SCAC)6.3.2026 22:42:00 CET | Press release
- Zynyz® (retifanlimab) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) is the first systemic treatment for adult patients with advanced SCAC in Europe- The EC approval is based on results of the POD1UM-303 study which showed that adult patients with advanced SCAC achieved significantly improved progression-free survival with Zynyz in combination with carboplatin and paclitaxel as a first-line treatment compared to chemotherapy alone.1 Incyte (Nasdaq:INCY) today announced that the European Commission (EC) has approved Zynyz® (retifanlimab) in combination with carboplatin and paclitaxel (platinum-based chemotherapy) for the first-line treatment of adult patients with metastatic or with inoperable locally recurrent squamous cell carcinoma of the anal canal (SCAC). “The EC approval of Zynyz marks an important step forward for patients with advanced SCAC, a rare cancer for which meaningful treatment advances have not occurred in several decades,” said Bill Meur
Dfns Launches Payouts6.3.2026 21:27:00 CET | Press release
Dfns today announced the launch of Payouts, a new API enabling institutions to convert stablecoins to fiat and route payouts across multiple bank accounts while keeping wallet-level governance and controls in place. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260305327930/en/ Convert stablecoins to fiat and settle payouts to bank accounts in 94 countries, today. Solving the problem of single-rail off-ramps Today, most fintechs and institutions still hard-wire a single payout provider into their stack, or rely on vertically integrated models that bundle routing, pricing, custody, and settlement together. That approach may be convenient early on, but it creates structural problems at scale: weak price discovery because there is no competitive pressure on margins, limited auditability because routing decisions are opaque, and operational fragility because a single provider degradation in any corridor requires architectural i
Klarna Group Plc Clarifies Mechanics of March 9 Lock-Up Expiration6.3.2026 20:23:00 CET | Press release
Klarna Group plc (NYSE: KLAR) today issues the following clarification to ensure investors and market participants have accurate information regarding the mechanics of its lock-up expiration on March 9, 2026, the processes required before pre-IPO shares can be traded on the NYSE, and the prior liquidity opportunities already available to shareholders. This release contains only factual descriptions of the Company's share structure and applicable processes. It does not constitute guidance or a projection of any kind regarding future trading volumes, share price, or the intentions of any shareholder and speaks only as of the date of this press release. 1. 335 million locked-up shares — but two different categories Of the 378 million total ordinary shares outstanding, approximately 335 million are subject to lock-up restrictions expiring March 9, 2026. However, these shares fall into two distinct categories governed by separate sets of regulations. A. 159 million shares (48% of locked-up
Lone Star Funds Announces Agreement to Acquire the Capsules & Health Ingredients Division of Lonza Group AG6.3.2026 18:30:00 CET | Press release
Lone Star Funds (“Lone Star”) today announced that an affiliate of Lone Star Fund XII, L.P. has entered into a definitive agreement to acquire the Capsules & Health Ingredients (“CHI”) division of Lonza Group AG. As part of the transaction, Lonza will retain a 40% equity position in the business. Headquartered in Basel, Switzerland, CHI operates globally across the Americas, Europe and Asia Pacific. The business comprises three segments: Hard Empty Capsules: leading global manufacturer of gelatin and plant-based capsules offering a broad range of innovative solutions for pharmaceutical and nutraceutical customers. Dosage Form Solutions: end-to-end development and manufacturing platform serving nutraceutical and pharmaceutical customers. Health Ingredients: provider of branded, science-backed nutrition ingredients serving joint health, energy and active lifestyle markets. Lone Star believes CHI is a high-quality, globally recognized platform with strong technical capabilities, different
In our pressroom you can read all our latest releases, find our press contacts, images, documents and other relevant information about us.
Visit our pressroom